An unknown gas effuses at a rate that is 0.462 times that of nitrogen gas (at the
Question:
An unknown gas effuses at a rate that is 0.462 times that of nitrogen gas (at the same temperature). Calculate the molar mass of the unknown gas in g/mol.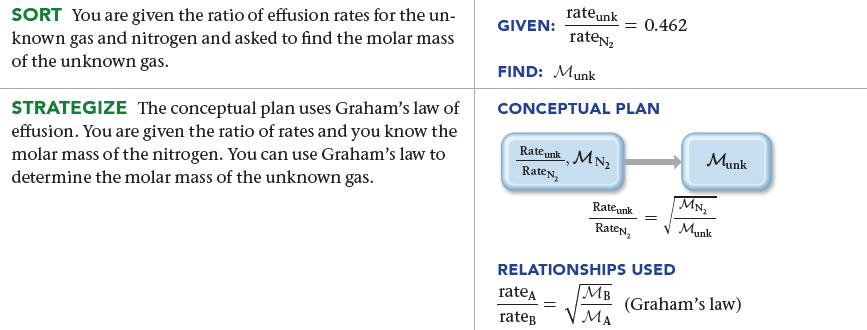
Transcribed Image Text:
SORT You are given the ratio of effusion rates for the un- known gas and nitrogen and asked to find the molar mass of the unknown gas. STRATEGIZE The conceptual plan uses Graham's law of effusion. You are given the ratio of rates and you know the molar mass of the nitrogen. You can use Graham's law to determine the molar mass of the unknown gas. GIVEN: rate unk rateN₂ FIND: Munk CONCEPTUAL PLAN Rate unk, MN₂ RateN₂ rateA rateg = 0.462 Rate unk RateN₂ MB H MA RELATIONSHIPS USED = Munk MN₂ √ Munk (Graham's law)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
rate unk rateN ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An unknown gas effuses at a rate that is 0.578 times that of nitrogen gas at the same temperature. Calculate the molar mass of the unknown gas in g/mol.
-
You have a gas, one of the three known phosphorusfluorine compounds (PF 3 , PF 5 , and P 2 F 4 ). To find out which, you have decided to measure its molar mass. (a) First, you determine that the...
-
If 4.83 mL of an unknown gas effuses through a hole in a plate in the same time it takes 9.23 mL of argon, Ar, to effuse through the same hole under the same conditions, what is the molecular mass of...
-
For a corporation such as Bell Canada, what are the two primary advantages of equity financing? Ownership is spread among many individuals, and no interest payments are required. Investors pay top...
-
A number of accounting journals now post back issues, or even publish their entire journals, online. Access the Journal of Accountancy website at www.aicpa.org (or another website selected by your...
-
An interaction overview diagram is a type of (a) deployment diagram (b) sequence diagram (c) interaction diagram (d) composite structure diagram
-
Will the organizational structure selected by the partners have an impact on your decision to extend the loan?
-
Using appropriate tables, solve the following future value of annuity problems: Required 1. What is the future value on December 31, 2013 of seven cash flows of $10,000, with the first cash payment...
-
Break Even Sales Under Present and Proposed Conditions Portmann Company, operating at full capacity, sold 1,000,000 units at a price of $190 per unit during the current year. The income statement is...
-
Twenty-five years ago, Angelo and Fred started their own consulting company, XYZ Co. Angelo, who is 55, retired from the business on December 31, 2019. He and his wife plan to travel throughout...
-
What is partial pressure? What is the relationship between the partial pressures of each gas in a sample and the total pressure of gas in the sample?
-
How does the density of a gas depend on temperature? Pressure? How does it depend on the molar mass of the gas?
-
For the single- period evolution in the preceding figure, what is the value of a floorlet with maturity time 1 and strike rate 0.03? Compute the value using risk- neutral valuation.
-
A company determines that monthly sales S(t), in thousands of dollars, after t months of marketing a product is given by S(t) = 23-551 + 230t+ 160. a) Find S'(1), S'(2), and S'(4). b) Find S''(1),...
-
Dan is a 16 year-old who decided to skip his adolescent development class. If Dan was 19 years-old, this would be his choice, but because of his age, he has broken the law. What type of offence did...
-
You need to remove a bolt from a metal door. The maximum torque the bolt can withstand before starting to rotate is 7 = 70 N-m. You apply a wrench of m = 0.5 kg and 1 = 0.3 m long. You push down on...
-
Lesson 10.1: Emotional Intelligence Emotional Intelligence is a type of social intelligence that affords the individual the ability to monitor his own and others' emotions, to discriminate among...
-
Harriet??s annuity has a total cash value of $2000, and she has paid a total of $1,500 into it. Under a Section 1035 exchange, Harriet rolls the entire value of the annuity into a different annuity....
-
Estimate the Brinell harnesses for specimens of a 0.45 wt% C iron-carbon alloy that have been subjected to the heat treatments described in parts (a), (d), and (h) of Problem 10.20. Problem 10.20 (a)...
-
You work as an operations consultant for a textile company. Your client has a well-established distribution system in the US market. The company has hundreds of stores and four distribution centers....
-
Determine the reactions at the supports, then draw the moment diagram. Assume A is a pin and B and C are rollers. EI is constant. 600 lb/ft 15 ft 15 ft
-
Determine the reactions at the supports, then draw the moment diagram. Assume the support at Bis a roller. EI is constant. 400 lb-ft - 8ft- 8 ft
-
The simply supported beam is subjected to the loading shown. Determine the deflection at its center C. EI is constant. 6 kip/ft 5 kip-ft A 8 ft- 8 ft
-
ignore question 3 just do bond A and Bond B the second photo Bond A: The $1,000 par value bond matures in 8 years, with a coupon rate of 10%. With one payment per year you estimate the Yield to...
-
The profitability of a deposit in euros expressed in dollars is: Select one: approximately the rate of appreciation of the dollar against the euro approximately the European market interest rate plus...
-
A disadvantage of balance sheet numbers is that assets reflect their book values. Select one: a. False b. True

Study smarter with the SolutionInn App


