Based on the phase diagram of CO 2 shown in Figure 12.39(b), describe the state changes that
Question:
Based on the phase diagram of CO2 shown in Figure 12.39(b), describe the state changes that occur when the temperature of CO2 is increased from 190 K to 350 K at a constant pressure of
(a) 1 atm,
(b) 5.1 atm,
(c) 10 atm, and
(d) 100 atm.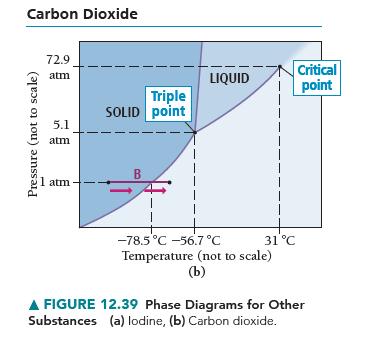
Transcribed Image Text:
Carbon Dioxide Pressure (not to scale) 72.9 atm 5.1 atm 1 atm Triple SOLID point B LIQUID -78.5°C -56.7 °C Temperature (not to scale) (b) 31 °C Critical point A FIGURE 12.39 Phase Diagrams for Other Substances (a) lodine, (b) Carbon dioxide.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a CO s b CO s above 216 K c CO...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What was the decision of the judicial body in answer to the issues of the case "Quesnelle v. Camus Hydronics Ltd., 2022"?
-
The phase diagram of sulfur is shown here. (a) How many triple points are there? (b) Monoclinic and rhombic are two allotropes of sulfur. Which is more stable under atmospheric conditions? (c)...
-
Question 3 A bullish signal is most likely sent by B a low debt/equity ratio. A a high P/E ratio. B a high good beta and a low bad beta. Question 4 a bullish candlestick with no upper shadow. How to...
-
Officer Cyril Rombach of the Burbank Police Department, an experienced and well-trained narcotics officer, applied for a warrant to search several residences and automobiles for cocaine,...
-
Consider a binary mixture of ethanol, EtOH (CH3CH2OH) and water (H2O) . A plot of the partial molar volumes of EtOH and H2O versus mole fraction EtOH (xEtOH) is provided in the following fi gure....
-
Why are precise research questions especially crucial in content analysis?
-
Summary information from the financial statements of two companies competing in the same industry follows. Required 1. For both companies compute the (a) Current ratio, (b) Acid-test ratio, (c)...
-
What should be the price of Oracle bond (in $)? Intro A Microsoft and an Oracle bond both have 14 years to maturity, a $1,000 par value, an AA rating and pay interest semiannually. Microsoft has a...
-
Three different analytic studies by three experienced and highly respected groups of researchers yielded three similar but somewhat different results. Do you believe that the results of these studies...
-
A sealed flask contains 0.55 g of water at 28 C. The vapor pressure of water at this temperature is 28.35 mmHg. What is the minimum volume of the flask in order that no liquid water be present in the...
-
Air conditioners not only cool air but dry it as well. A room in a home measures 6.0 m * 10.0 m * 2.2 m. If the outdoor temperature is 30 C and the partial pressure of water in the air is 85% of the...
-
How many of the String objects are eligible for garbage collection right before the end of the main() method? A. None B. One C. Two D. Three E. None of the above public static void main(String[]...
-
Pink Jeep Tours offers off-road tours to individuals and groups visiting the Southwestern U.S. hotspots of Sedona, Arizona, and Las Vegas, Nevada. Take a tour of the companys Web site at...
-
The following are unrelated accounting practices: 1. Pine Company purchased a new \(\$ 30\) snow shovel that is expected to last six years. The shovel is used to clear the firm's front steps during...
-
Identify whether the following statements are true or false. 1. One argument for IFRS is that there is less globalization in the world. 2. IFRS is accepted as GAAP in every country of the world. 3....
-
You will need isometric dot paper for this question. Part of a pattern using four rhombuses is drawn on isometric dot paper below. By drawing two more rhombuses, complete the pattern so that it has a...
-
Fred Flores operates a golf driving range. For each of the following financial items related to his business, indicate the financial statement (or statements) in which the item would be reported:...
-
Write equations for the following reactions: a. 2-octyne + H2 (1 mole, Lindlar's catalyst) b. 3-hexyne + Br2 (2 moles) c. 1-hexyne + sodium amide in liquid ammonia d. 1-butyne + H2O (H+, Hg2+...
-
If the jobs displayed in Table 18.24 are processed using the earliestdue-date rule, what would be the lateness of job C? TABLE 18.24 Processing Times and Due Dates for Five Jobs Job C D E...
-
Identify the two aldohexoses that are obtained when d-arabinose undergoes a Kiliani-Fischer synthesis.
-
Identify the two products obtained when d-glyceraldehyde is treated with HCN, and determine the relationship between these two products.
-
When treated with sodium borohydride, d-glucose is converted into an alditol. (a) Draw the structure of the alditol. (b) Which l-aldohexose gives the same alditol when treated with sodium borohydride?
-
8. Implied interest rate and period Aa Aa Consider the case of the following annuities, and the need to compute either their expected rate of return or duration Jacob needed money for some unexpected...
-
Assuming a UK investor holds the following 8 assets in the portfolio. 1. GME shares 2. S&P 500 3. Vanguard S&P 500 ETF 4. Bitcoin 5. Samsung bond 6. cash in foreign currency (USD) 7. UK government...
-
Select one major economic indicator from either interest rates, unemployment, GDP, or inflation. What is the current level of this indicator and make a prediction for how this will move over the next...

Study smarter with the SolutionInn App


