Calculate the density of nitrogen gas at 125 C and a pressure of 755 mmHg. SORT The
Question:
Calculate the density of nitrogen gas at 125 °C and a pressure of 755 mmHg.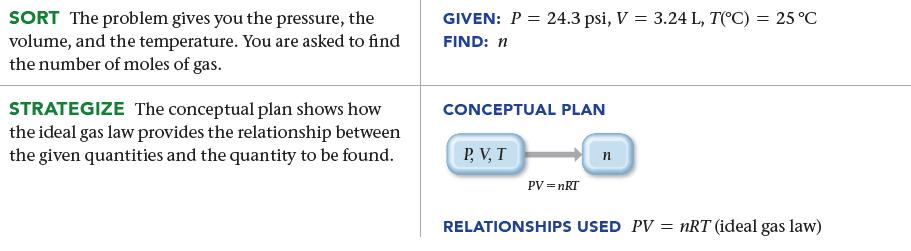
Transcribed Image Text:
SORT The problem gives you the pressure, the volume, and the temperature. You are asked to find the number of moles of gas. STRATEGIZE The conceptual plan shows how the ideal gas law provides the relationship between the given quantities and the quantity to be found. GIVEN: P = 24.3 psi, V = 3.24 L, T(°C) = 25 °C FIND: n CONCEPTUAL PLAN PV, T PV = nRT RELATIONSHIPS USED PV = nRT (ideal gas law)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
TK 125 273 P 755 mmHg x ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A sample of gas has a mass of 0.311 g. Its volume is 0.225 L at a temperature of 55 C and a pressure of 886 mmHg. Find its molar mass. SORT The problem gives you the temperature and pressure of...
-
A sample of nitrogen gas at 18oC and 760 mmHg has a volume of 3.92 mL. What is the volume at 0oC and 1 atm of pressure?
-
Calculate the density of hydrogen bromide (HBr) gas in grams per liter at 733 mmHg and 46C.
-
Which description does NOT fit in description of "issues" in the context of international standards? a. An unsettled matter. b. A vital matter. c. A change in the environment. d. A concern or...
-
Visit www.isaca.org, the website for the Information Systems Audit and Control Association, and examine two case studies of organizations that use COBIT. Explain how these entities obtain value by...
-
According to an article in the Wall Street Journal in mid-2019, cash hasnt completely disappeared yetan attempt to pay with Venmo . . . will be met with a bemused shake of the head at plenty of...
-
Can there be too much information in managing the business of baseball? Discuss.
-
Using the data in E12-3, assume that Slick Books management purchased the Syntax stock for the trading securities portfolio instead of the securities available for sale portfolio. Prepare any journal...
-
Amos reyes can now coon The Changement Dies and its est month of operations in 2015 Click to contactos Road Des Requirements were fiets of the events that of Ames Sin CPA Analythe events chronopoly...
-
In Florida, real estate agents refer to homes having a swimming pool as pool homes. In this case, Sunshine Pools Inc. markets and installs pools throughout the state of Florida. The company wishes to...
-
Explain why people may experience ear pain after a rapid change in altitude.
-
A 255-mL gas sample contains argon and nitrogen at a temperature of 65 C. The total pressure of the sample is 725 mmHg, and the partial pressure of argon is 231 mmHg. What mass of nitrogen is present...
-
State the Divergence Theorem.
-
Question 37 Plantito Inc., produces potted plants. For next year, Pietro predicts that 45,000 units will be produced, with the following total costs: Direct materials Direct labor ? 80,000 Variable...
-
When you are to design a data transmission system, you have two key considerations to work with: data rate and distance, with emphasis placed on achieving the highest data rates over the longest...
-
How much work does a supermarket checkout attendant do on a can of soup he pushes 0.600 m horizontally with a force of 5.00 N? Express your answer in joules and kilocalories. 3 . (a) Calculate the...
-
Suppose in its income statement for the year ended June 30, 2022, The Clorox Company reported the following condensed data (dollars in millions). Salaries and wages expenses$460 Research and...
-
Consider the extensive form game show in the figure below. How many strategies does Player 2 have in this game? (2,2,1) b (2,4,2) 03 by 03 02 dz (4.2,0) (2.0.2) (0.3.4) (3,5,3) (3,1,2)
-
Determine values for the constants n and k (Equation 10.17) for the recrystallization of copper at 102C.
-
Draw two scatterplots, one for which r = 1 and a second for which r = 21.
-
Determine the stiffness matrix K for the truss. Take A = 0.75 in 2 , E = 29(10 3 ) ksi. 2 500 lb 4 ft (3) -3 -31H 4 ft-
-
Determine the vertical displacement at joint ¡ and the force in member ¤. Take A = 0.0015 m 2 and E = 200 GPa. 2 m 2 m- 4 10 2 m 4 30 kN
-
Determine the force in member ¤. Take A = 0.0015 m 2 and E = 200 GPa for each member. 410 44 42 3 20 kN 3 m -4 m- 4 m 3.
-
ABC Corp currently has a debt to enterprise value ratio of 51%. The firm's cost of equity is 8.4% and its cost of debt is 4%. Assuming perfect markets, calculate the unlevered cost of capital for ABC...
-
Rebound Airlines was hurt in the recession, with its stock falling to $10 from $50. The stock has started to recover and has just crossed its 50-day moving average at $20. The 200-day moving average...
-
Biotech Corp has no sales or earnings but is working on a COVID cure. They also have some good prospects for other drugs that could potentially be blockbusters. The stock started the year at $30 and...

Study smarter with the SolutionInn App


