Closely examine the structure of glucose shown here. Does glucose exhibit optical isomerism (discussed in Section 22.3)?
Question:
Closely examine the structure of glucose shown here. Does glucose exhibit optical isomerism (discussed in Section 22.3)? If so, which carbon atoms are chiral?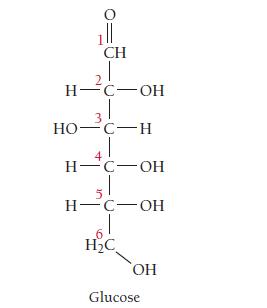
Transcribed Image Text:
1ll CH H-C-OH 3 HO-C-H H-C-OH H-C-OH H2C OH Glucose
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Any carbon atom with four different substituents att...View the full answer

Answered By

Stacy kosgei
I offer quality, original and timely services; Highly credible and void of plagiarism. Your success is my pleasure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Examine the structure of deoxyribose, part of the backbone in DNA. Does deoxyribose exhibit optical isomerism? If so, which carbon atoms are chiral? - - C-
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Examine the structure of the following experimental design. Determine which of the three designs presented in the chapter would be most likely to characterize this structure. Discuss the variables...
-
Let S be the portion of the cylinder y = e x in the first octant that projects parallel to the x-axis onto the rectangle R yz : 1 y 2, 0 z 1 in the yz-plane. Let n be the unit vector normal to S...
-
Prepare a bank reconciliation from the following information: a. Balance per bank statement as of June 30, $4,862.77 b. Balance per books as of June 30, $2,479.48 c. Deposits in transit, $654.24 d....
-
The DBA at Premiere Products wants you to investigate biometric identification techniques for potential use at the company for computer authentication purposes. Use books, articles, and/or the...
-
What challenges can Barrett expect in its export drive? What types of new capabilities does the firm need to acquire to manage its export transactions? LO.1
-
Kirk Van Houten, who has been married for 23years, would like to buy his wife an expensive diamond ring with a platinum setting on their 30-year wedding anniversary. Assume that the cost of the ring...
-
A project has annual cash flows of $4000 for the next 8 years and $8000 each year for the following 10 years. the IRR of this 18-year project is 8%. if the firms WACC is 7%, what is the projects NVP?...
-
Which compound is a lipid? a) HO b) c) CH H C I - OH OH H3C-(CH2)12 0 || OH 1 H2N - C - C-OH T CH T CH I S I CH3 OH d) H3C-CH2-0-CH2-CH3
-
Which compound is most likely to have a foul odor? a) b) CH3CH, NH, c) CH3-CH-0-CH3 d) CH3-CH-CH-OH CH3-C-0-CH3
-
Consider Supplemental Exercise 10-85. Suppose that the true difference in mean fill volume is as much as 2 fluid ounces; did the sample sizes of 10 from each vineyard provide good detection...
-
Q1. (a) Name the types of reactions that organic compounds undergo (b) Differentiate between (i) electrophile and nucleophile
-
CH4 Br, Ligtht Q2. (a) CH3Br + HBr Propose a mechanism for the reaction; indicating initiation, propagation and termination.
-
Q4. Complete the following reactions by drawing the structure(s) of the product(s) formed.
-
1. Why did the Iconoclast emperors believe that using images in worship was wrong? 2. How are recent examples of iconoclasm similar to those of the early medieval period? 3. Why is iconoclasm a...
-
1. Difference Between Essential and Non-Essential Nutrients 2. what is Conditionally Essential Nutrients? explain with examples
-
Nautical Marina needs to raise $1.0 million to expand the company. Nautical Marina is considering the issuance of either $1,000,000 of 8% bonds payable to borrow the money, or 100,000 shares of...
-
Linda Lopez opened a beauty studio, Lindas Salon, on January 2, 2011. The salon also sells beauty supplies. In January 2012, Lopez realized she had never filed any tax reports for her business and...
-
A 6.8C charge and a -4.7C charge are inside an uncharged sphere. Whats the electric flux through the sphere?
-
A 2.6C charge is at the center of a cube 7.5 cm on each side. Whats the electric flux through one face of the cube? (Think about symmetry, and dont do an integral.).
-
The electric field at the surface of a 5.0-cm-radius uniformly charged sphere is 90 kN/C. Whats the field strength 10 cm from the surface?
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


