Complete and balance each equation. If no reaction occurs, write NO REACTION. a. NaNO3(aq) b. NaCl (aq)
Question:
Complete and balance each equation. If no reaction occurs, write “NO REACTION.”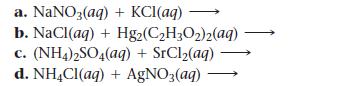
Transcribed Image Text:
a. NaNO3(aq) b. NaCl (aq) + c. (NH4)2SO4(aq) + SrCl₂(aq) d. NH4Cl(aq) + AgNO3(aq) + KC1(aq) Hg2(C₂H3O2)2(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
here are the completed and balanced e...View the full answer

Answered By

Vineet Kumar Yadav
I am a biotech engineer and cleared jee exam 2 times and also i am a math tutor. topper comunity , chegg India, vedantu doubt expert( solving doubt for iit jee student on the online doubt solving app in live chat with student)
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Complete and balance each of the following molecular equations, including phase labels, if a reaction occurs. Then write the net ionic equation. If no reaction occurs, write NR after the arrow. a....
-
Complete and balance each of the following molecular equations, including phase labels, if a reaction occurs. Then write the net ionic equation. If no reaction occurs, write NR after the arrow. a....
-
Complete and balance each of the following equations. If no reaction occurs, write NOREACTION. Express your answer as a chemical equation. Identify all of the phases in your answer. 1) KCl (aq) + CaS...
-
Consider : You have been asked to evaluate whether your organization's current pay structure makes sense in view of what competing - address the following: How would you determine what organizations...
-
What company policies or procedures would you recommend to prevent each of the following activities? a. A clerk at the Paul Yelverton Company faxes a fictitious sales invoice to a company that...
-
Indicate whether each of the following costs would be expensed (E) or capitalized (C) under full cost (FC) and successful efforts (SE) accounting. Cost a. Aerial magnetic study-an area of interest is...
-
Many professional hospitality associations provide helpful training resources. Check out the Web sites for the following organizations to review the types of training materials, resources, and...
-
Bangkok Instruments, Ltd., the Thai subsidiary of a U.S. corporation, is a seismic instrument manufacturer. Bangkok Instruments manufactures the instruments primarily for the oil and gas industry...
-
Mcknight, Inc. manufactures bookcases and uses an activity-based costing system. McKnight's activity areas and related data follow: McKnight produced two styles of bookcases in October: the standard...
-
You have been asked to prepare the financial statements for Computer Solutions for the year ended December 31, 2021. The following additional facts are collected for use in making adjusting entries...
-
Write a molecular equation for the precipitation reaction that occurs (if any) when each pair of aqueous solutions is mixed. If no reaction occurs, write NO REACTION. a. Potassium carbonate and...
-
Complete and balance each equation. If no reaction occurs, write NO REACTION. a. Lil(aq) + BaS(aq) b. KCl(aq) + CaS(aq) c. CrBr(aq) +NaCO3(aq) d. NaOH(aq) + FeCl3(aq)
-
A company should make provision for which one of the following? (a) Present obligation on account of obligating events (b) For future operating expenses (c) For future contracts (d) All of these (e)...
-
Based on a survey, assume that 42% of consumers are comfortable having drones deliver their purchases. Suppose that we want to find the probability that when six consumers are randomly selected,...
-
What is the social location that determines this speech community? Is it determined by race, class, gender, sexuality, or some other social location? What makes this speech community unique? What are...
-
Write a program named SumOfNumberOfSquares.java that prompts user to enter a number of integers and calculates the sum of their squares. The following is a sample run. The green fonts represent user...
-
6.4 Charles Augustin de Coulomb was a French physicist who is best known for formulating the law that calculates the force between two electric charges. To honor Coulomb, the unit of electric charge...
-
What amount of cash payments to suppliers will be reported by Indigo Company for the year ended December 31, 2024?
-
A polystyrene component must not fail when a tensile stress of 1.25 MPa (180 psi) is applied. Determine the maximum allowable surface crack length if the surface energy of polystyrene is 0.50 J/m2...
-
1) Predict the organicproduct formed when BzCl reacts with cyclohexanol. BzCl = benzoylchloride. 2) Provide the majororganic product of the reaction below. 3) Draw the structureof the product formed...
-
Calculate the mass of 1 ft 3 of gasoline if it weighs 42.0 lb.
-
Calculate the weight of 1 ft 3 of kerosene if it has a mass of 1.58 slugs.
-
Calculate the weight of 1 gal of water if it has a mass of 0.258 slug.
-
This table shows the stock returns for Stock A and Stock B in three different scenarios. The first column shows how likely each of the three scenarios is. The risk-free rate is 5%. Calculate the...
-
The STI Desk Company manufactures student desks that it sells for $80 per unit. Current cost information is as follows: Variable Costs: Direct Material per Desk - $20 Direct Labour per Desk - $15...
-
8. Implied interest rate and period Aa Aa Consider the case of the following annuities, and the need to compute either their expected rate of return or duration Jacob needed money for some unexpected...

Study smarter with the SolutionInn App


