Consider the reaction: A reaction mixture at 100 C initially contains [NO 2 ] = 0.100 M.
Question:
Consider the reaction: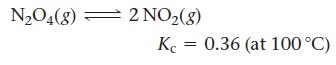
A reaction mixture at 100 °C initially contains [NO2] = 0.100 M. Find the equilibrium concentrations of NO2 and N2O4 at this temperature.
Transcribed Image Text:
N₂O4(8) = 2 NO₂(8) Kc 0.36 (at 100 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
NO48 2 NOg Initial Change Equil Qc NO N04 N04 NO 000 0100 0100 000 00 Q ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Mr. Creary has hired you to estimate a selling price for his house using the direct market comparison method. You have compiled the following information: Features Sale Price Sale Time Lot Size House...
-
Consider the reaction: 2 H 2 S (g) 2 H 2 (g) + S 2 (g) Kc = 1.67 10 -7 A reaction mixture initially contains [H 2 S] = 0.010 M. Find the equilibrium concentrations of H 2 and S 2 .
-
Player 1 and Player 2 are involved in a dispute. Player 2 is either Weak or Strong. Player 2's strength is private information. 2 knows his strength; 1 knows that 2 is Strong with probability p=0.35...
-
A project is at the multi-functional planning phase. The project manager realizes that producing a complex application is not feasible with the current number of resources and decides to hire more...
-
What is a focused value stream?
-
Insurance companies never know the exact amounts of their future payouts. So, why do they hold large amounts of long-term, relatively illiquid assets, such as corporate bonds, that may be difficult...
-
Determine the message you wish to convey to applicants.
-
Information about Lindas Boards is presented in E6-4. Additional data regarding Lindas sales of Xpert snowboards are provided below. Assume that Lindas uses a perpetual inventory system. In E6-4,...
-
Problem 6-6B Record transactions using a perpetual system, prepare a partial income statement, and adjust for the lower of cost and net realizable value(LO6-2, 6-3, 6-4, 6-5, 6-6) [The following...
-
The DSV Partnership decided to liquidate as of June 30, 20X5. Its balance sheet as of this date follows: DSV PARTNERSHIP Balance Sheet At June 30, 20xs Assets $ 50,000 95,000 75,000 Cash Accounts...
-
Does the value of the equilibrium constant depend on the initial concentrations of the reactants and products? Do the equilibrium concentrations of the reactants and products depend on their initial...
-
For the reaction, A(g) 2 B( g), K c = 4.0. A reaction mixture at equilibrium contains [A] = 1.0 M. What is the concentration of B in the reaction mixture? (a) 0.50 M (b) 1.0 M (c) 2.0 M (d) 4.0 M
-
What is value-engineering, and what role does it play in target costing?
-
Explain at least 8 types of Google ads brieflyAnalyze the ad & share your opinion on its performance and suggest changes if required. * add the snapshots, and pictures of examples
-
Categorize each variable as quantitative or qualitative GPA is continuous Number of students is Discrete GPA ( Continuous) and Number of Students ( Discrete) GPA ( Discrete) and the Number of...
-
Silver Company makes a product that is very popular as a Mothers Day gift. Thus, peak sales occur in May of each year, as shown in the companys sales budget for the second quarter given below: April...
-
Among the following statements, select the ones which have a positive environmental impact. Choose several answers Minimising the impact of a product on the environment Avoiding the destruction of a...
-
Developing Financial Statements: All organizations, including those in the healthcare industry, need to make money to be profitable and survive. Financial statements, such as balance sheets, profit...
-
We consider the random process St, which plays a fundamental role in Black-Scholes analysis: where Wt is a Wiener process with W0 = 0, is a "trend" factor, and (Wt Ws) N (0, (t s)) which says...
-
Suppose that the laptop of Prob. 2.16 is placed in an insulating briefcase with a fully charged battery, but it does not go into sleep mode, and the battery discharges as if the laptop were in use....
-
What is the basic distinction between the scientific method and other ways of looking at the natural world?
-
Scientific models do not correspond exactly to reality. Why are they nevertheless so useful?
-
What does a year correspond to in terms of observations of the sun and stars?
-
Suppose a smoker indicates on an application for medical expense insurance that he is a nonsmoker. Is this misrepresented material?
-
Question 10. What is the effect of traders storing grain to wait for higher prices? 1. It is essential in preventing grain shortages. 2. Traders are able to monopolize the market. 3. Most shortages...
-
Differentiate between the profit and loss from writing a covered call option versus a naked call option

Study smarter with the SolutionInn App


