Consider the reaction: A reaction mixture at 2000 C initially contains [N 2 ] = 0.200 M
Question:
Consider the reaction:
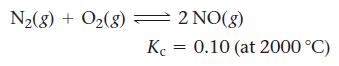
A reaction mixture at 2000 °C initially contains [N2] = 0.200 M and [O2] = 0.200 M. Find the equilibrium concentrations of the reactants and product at this temperature.
Transcribed Image Text:
N₂(8) + O₂(8) 2NO(g) Kc = 0.10 (at 2000 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Initial Change Equil Qc N2g O28 2 NOg N 0 0200 0200 NO N0 000 ...View the full answer

Answered By

Yadram Dhanka
I was engaged in conducting private tuitions for students of class 11th and 12th. I would like to work with a leading educational organization and to use my in-depth subject knowledge and passion towards teaching to the best of my ability, so as to enrich the student’s ability to learn, as well as to advance my career in the education sector.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Initially a mixture contains 0.795 mol each of N2 and O2 in an 8.00-L vessel. Find the composition of the mixture when equilibrium is reached at 3900oC. The reaction is and Kc = 0.0123 at 3900oC....
-
A dependent is any individual who received more than half of his or her support for the most recent calendar year from the relevant individual (i.e. the KPMG member firm professional, his or her...
-
A reaction chamber contains a mixture of CO2, CO, and O2 in equilibrium at a specified temperature and pressure. Now some N2 is added to the mixture while the mixture temperature and pressure are...
-
What are the Four Eras of Commercial Aviation Safety in order from the 1950's to the future?
-
How are value streams identified and created?
-
Suppose that Apples profits are expected to grow twice as fast as Microsofts. Which firms stock should be the better investment for you? Briefly explain.
-
How will we communicate to employees their progress and success?
-
Production and purchases budgets. Osage Inc. has actual sales for May and June and forecast sales for July, August, September, and October as follows: Actual: May . . . . . . . . . . . . . . . . . ....
-
What was ABC's operating cash flow in To access ABC's income statement, see below: 1000 200 50 750 Revenues or Sales Expenses Depreciation Expenses EBIT Interest Expense EBT Tax Net Income 30 720 151...
-
Prevosti Farms and Sugarhouse pays its employees according to their job classification. The following employees make up Sugarhouse's staff: Employee Number Name and Address Payroll information...
-
Why are the concentrations of solids and liquids omitted from equilibrium expressions?
-
For the reaction N 2 O 4 (g) 2 NO 2 (g), a reaction mixture at a certain temperature initially contains both N 2 O 4 and NO 2 in their standard states, which means that P N2O 4 = 1 atm and P NO 2 =...
-
TV viewers cannot receive scrambled signals unless they have a decoder. Whoever receives digital satellite signals receives scrambled signals. Therefore, whoever receives digital satellite signals...
-
Cogenesis Corporation is replacing their current steam plant with a 6-megawatt cogeneration plant that will produce both steam and electric power for their operations. What is the impact of a 5% and...
-
Suppose ABC firm is considering an investment that would extend the life of one of its facilities for 5 years. The project would require upfront costs of $9.97M plus $28.94M investment in equipment....
-
Which one of the following therapists' approaches has been integrated into several other therapies in the West? 1. Naikan therapy 2. Morita therapy 3. mindfulness meditation
-
What does this scatter plot tell us? Check ALL below that are true from the Scatter Plot. Y is cumulative total barrels and x is number of active wells. 1. If there were 550 active wells, we would...
-
Millie runs a small company that makes customised notebooks with personalised details on the cover and inserts. You promote your product as a great gift idea, and your holiday orders break your...
-
You are given the representation: where the equality holds, given the sequence of information sets {It}. The underlying process Xt is known to follow the SDE: dXt = μdt + ÏdWt...
-
In Problem use geometric formulas to find the unsigned area between the graph of y = f(x) and the x axis over the indicated interval. f(x) = x + 5; [0, 4]
-
For the circuit in Fig. 5.93 , find v o . 25 k2 100 k2 40 k2 20 k2 20 k2 10 k2 Vo +, 2 V 0+
-
Find v o in the op amp circuit of Fig. 5.92 . 100 k2 www 100 k2 10 k2 100 k2 30 2 30 k2 +. ww- 70 mv 70 kQ Vo Q+
-
Determine v o in the circuit of Fig. 5.88 . 20 k2 -0.2 V 10 k2 40 k2 1.2 V 10 k2 20 k2
-
Use the future value formula to find the indicated value. n=20; i = 0.03; PMT = $80; FV = ? FV=$1 (Round to the nearest cent.)
-
An unlevered firm has an EBIT = $250,000, aftertax net income = $165,000, and a cost of capital of 12%. A levered firm with the same assets and operations has $1.25 million in face value debt paying...
-
Suppose Mike Inc. has 100 shares outstanding. It receives a constant net income of $1,000 annually and will pay all of it as dividends. What is the stock price today? Assuming the required rate of...

Study smarter with the SolutionInn App


