Consider the reaction for the decomposition of hydrogen disulfide: A 0.500-L reaction vessel initially contains 0.0125 mol
Question:
Consider the reaction for the decomposition of hydrogen disulfide: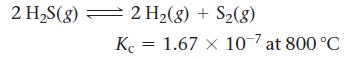
A 0.500-L reaction vessel initially contains 0.0125 mol of H2S at 800 °C. Find the equilibrium concentrations of H2 and S2.
Transcribed Image Text:
2 H₂S(g) → 2 H₂(g) + S₂(8) Kc = 1.67 x 107 at 800 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
HS 00125 mol 0500 L Initial Change Equil 00250 M 2 HSg 2 Hg S28 HS H 00250 000 S 00...View the full answer

Answered By

Kennedy Odhiambo
As a professional writer, I have been in the field for over 5 years having worked as a lecture in different tertiary institutions across the world. With this impeccable experience, I assure provision of a good and supporting environment for students to learn.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the reaction for the decomposition of hydrogen disulfide: A 0.500-L reaction vessel initially contains 1.25 * 10 -4 mol of H 2 S at 800 C. Find the equilibrium concentrations of H 2 and S 2...
-
The following equilibrium was studied by analyzing the equilibrium mixture for the amount of H2S produced. A vessel whose volume was 2.50 L was filled with 0.0100 mol of antimony(III) sulfide, Sb2S3,...
-
At some temperature, a 100-L reaction vessel contains a mixture that is initially 1.00 mol CO and 2.00 mol H2. The vessel also contains a catalyst so that the following equilibrium is attained: At...
-
Assume that a company is going to invest 900,000 USD in a new project. We expect that the invested capital in the fixed assets will be fully depreciated within 3 years as follows: 500,000 USD,...
-
Pollard Pharm, Inc., has the following departmental structure for producing a popular pain medication: A consultant designed the following cellular manufacturing structure for the same product: The...
-
Current and projected free cash flows for Radell Global Operations are shown below. Growth is expected to be constant after 2015, and the weighted average cost of capital is 11%. What is the horizon...
-
What are the advantages and disadvantages of internal recruiting?
-
Sanderson Companys year-end balance sheets follow. Express the balance sheets in common-size percents. Round amounts to the nearest one-tenth of a percent. Analyze and comment on theresults. At...
-
Q 1 / B - The following are information on three cases: Required / For each case determine flexible budget variance, favorable or unfavorable.
-
Does memory performance differ based on study format (word, picture, and auditory study styles) and/or test format (in person vs. online)? Students in a class (N = 36) were randomly assigned to one...
-
What is the definition of the reaction quotient (Q) for a reaction? What does Q measure?
-
The reaction X 2 (g) 2 X(g) occurs in a closed reaction vessel at constant volume and temperature. Initially, the vessel contains only X 2 at a pressure of 1.55 atm. After the reaction reaches...
-
The UNIX command ls can be used to view the ownerships and permissions of a file. What will be the output of the command ls l myStore | cut d' 'f1 if myStore is a file with protection mode...
-
Product costs using activity rates Body-Solid Inc. manufactures elliptical exercise machines and treadmills. The products are prouced in its Fabrication and Assembly production departments. In...
-
How to start an essay on the multigenerational workforce and your experiences working with each of the generations. Begin your essay with an introduction that outlines the current generations in the...
-
1. What does the phrase "cost of quality" mean? How might using this statement assist a company in addressing its quality issues? 2. What key distinctions exist between total quality human resource...
-
Does productivity in terms of output per labor our insure a company will be profitable? Why or why not? What questions should be asked to test whether productivity has increased? How do these answers...
-
How do the four Ps of marketing (product, price, promotion, place) differ in international markets?
-
Show that given t0, t1, . . . , tn1, tn and Wt0 ,Wt1 , . . . ,Wtn1 ,Wtn we can always write: How is this different from the standard formula for the differentiation of products: d(uv) = (du)v + u(dv)...
-
A Bloomberg Businessweek subscriber study asked, In the past 12 months, when traveling for business, what type of airline ticket did you purchase most often? A second question asked if the type of...
-
Determine v 1 and v 2 in the circuit of Fig. 3.71 . 10 10 V1 V2 + v. 10 20 10 V (+ 5
-
Use nodal analysis to find V o in the circuit of Fig. 3.72 . 3V 30 60 30 . 30 V 60 +1
-
Use mesh analysis to obtain i a , i b , and i c in the circuit in Fig. 3.84 . 5 20 la 10 : 15 30 V(+ 45 V
-
Rod will use the single filing status when he files his 2020 return. Most of his income is from wages, but he does have a capital gain of $5,000 from the sale of stock. His adjusted gross income of...
-
(Present value)Sarah Wiggum would like to make a single investment and have $2.2 million at the time of her retirement in 35 years. She has found a mutual fund that will earn 7 percent annually. How...
-
Fraudulent financial reporting is an intentional misstatement or omission of amounts or disclosures with the intent to deceive users. Select one: True False

Study smarter with the SolutionInn App


