Consider the reaction: If a solution initially contains 0.210 M HC 2 H 3 O 2 ,
Question:
Consider the reaction:
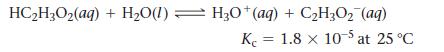
If a solution initially contains 0.210 M HC2H3O2, what is the equilibrium concentration of H3O+ at 25 °C?
Transcribed Image Text:
HC₂H3O₂ (aq) + H₂O(1) H3O+(aq) + C₂H3O₂ (aq) K 1.8 x 10-5 at 25 °C =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
19...View the full answer

Answered By

Navashree Ghosh
I believe in quality work and customer satisfaction. So, I can assure you that you will get quality work from me when you hire me. Let's work together and build a long-term association.
4.90+
82+ Reviews
116+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A chemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. The drug molecules bind the protein in a...
-
Compare and contrast Taylorism, Theory of Constraint, and Toyota Production System
-
The solution containing no added KNO3 for Figure 7-1 contains 5.0 mM Fe(NO3)3, 5.0 M NaSCN, and 15 mM HNO3. We will use Davies activity coefficients to find the concentrations of all species in the...
-
A ball is attached to one end of a wire, the other end being fastened to the ceiling. The wire is held horizontal, and the ball is released from rest (see the drawing). It swings downward and strikes...
-
What is the purpose of a base period?
-
Download the levi learning module leviRTS from the levi web site [497]. Model a job set as described in Table 4.1. tP,P and t P,C are the times relative to the start times, at which a job requests...
-
The following information was extracted from the 1997 financial report of the Generic Clothing Company. 1997 1996 Current assets: Cash $ 15,000 $ 30,000 Short-term marketable securities 225,000...
-
Coldplay Corporation incurred the following costs while manufacturing its product. Work-in-process inventory was $12,000 at January 1 and $15,500 at December 31. Finished goods inventory was $60,000...
-
A stock's returns have the following distribution: Demand for the Company's Products Probability of This Demand Occurring Rate of Return If This Demand Occurs Weak 0.1 (42%) Below average 0.3 (7)...
-
According to the SPSS output, what percentage of the variance in health knowledge is explained by age and academic knowledge? Assume that age and academic knowledge (graded exam: 0100%) have been...
-
Consider the reaction: If a reaction mixture initially contains 0.175 M SO 2 Cl 2 , what is the equilibrium concentration of Cl 2 at 227 C? SOCl(g) = SO(g) + Cl(g) K 2.99 x 10-7 at 227 C =
-
For the reaction shown here, K c = 255 at 1000 K. CO(g) + Cl 2 (g) COCl 2 (g) If a reaction mixture initially contains a CO concentration of 0.1500 M and a Cl 2 concentration of 0.175 M at 1000 K,...
-
Find examples to show that if lim f(x)g(x) lim f(x or lim g[:x
-
Suppose the city is undergoing severe ination. Specifically, both goods prices have risen by 10%. What percentage of a raise in the wage rate should Alex request from her boss, for her to maintain...
-
1. An iron cube of mass 0.55 kg is raised to a temperature of 100C by being placed in boiling water for 5 minutes. It is then removed and transferred immediately to an aluminium calorimeter filled...
-
Question 7 Two objects, of masses 3 and 4 kg, are hung from the ends of a stick that is 70 cm long and has marks every 10 cm, as shown above. If the mass of the stick is negligible, at which of the...
-
Since they do not have enough saved, Rachel and John would like to consider retiring later. Create a new timeline and recalculate all of the relevant values to determine at what age Rachel and John...
-
Problem 6 Find the partial derivative with respect to x for the following functions: (a) p = 56 (b) y(x)=56-4x (c) m = r (d) q= x (e) f(x) =x3 (f) g(x,y) = xy 2 (g) h(x,y) = Ax1/2y1/2, where A is a...
-
Show that the price of a swap is the same as the price of a cap minus the price of a floor.
-
Pedro Bourbone is the founder and owner of a highly successful small business and, over the past several years, has accumulated a significant amount of personal wealth. His portfolio of stocks and...
-
A pipeline is needed to transport medium fuel oil at 77F. The pipeline needs to traverse 80 mi in total, and the initial proposal is to space pumping stations 2 mi apart. The line needs to carry 750...
-
Soccer on a windy day. A professional soccer player can kick a ball with a speed of about 30 m/s (about 75 mi/h). Does air drag play a significant role in the trajectory of the ball? Give a reason...
-
When airplanes land or take off, they always travel along a runway in the direction that is into the wind because the lift force on an airplane wing depends on the speed of the airplane relative to...
-
4. President and Chairman of Sky Company plan to have the company issue $700 million of new equity and use the proceeds to pay off some of its outstanding debts. Assume that the company, which does...
-
Marlin Motors sells a single product with a selling price of $490 with variable costs per unit of $196. The company's monthly fixed expenses are $52,920. A. What is the company's break-even point in...
-
The return on the pension fund impacts the employers periodic pension expense for defined contribution pension plans. True or False

Study smarter with the SolutionInn App


