Determine the type of reaction (substitution, dehydration, oxidation, or reaction with an active metal) that occurs in
Question:
Determine the type of reaction (substitution, dehydration, oxidation, or reaction with an active metal) that occurs in each case, and write formulas for the products.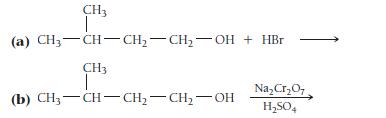
Transcribed Image Text:
CH3 (a) CH3-CH-CH₂-CH₂-OH + HBr CH3 I (b) CH₂-CH-CH₂-CH₂-OH Na₂Cr₂O7 H₂SO4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a An alcohol reacting with an acid is an example of a substitution reaction The product of the s...View the full answer

Answered By

Anjali Arora
Having the experience of 16 years in providing the best solutions with a proven track record of technical contribution and appreciated for leadership in enhancing team productivity, deliverable quality, and customer satisfaction. Expertise in providing the solution in Computer Science, Management, Accounting, English, Statistics, and Maths.
Also, do website designing and Programming.
Having 7 yrs of Project Management experience.
100% satisfactory answers.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Potassium metal burns in an atmosphere of chlorine gas. (b) Strontium oxide is added to water. (c) A fresh...
-
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Chlorine reacts with water. (b) Barium metal is heated in an atmosphere of hydrogen gas. (c) Lithium reacts...
-
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Cesium is added to water. (b) Strontium is added to water. (c) Sodium reacts with oxygen. (d) Calcium reacts...
-
Consider the heat pump described in Example 9.14. The heat pump now operates between 0.60 MPa and 1.4 MPa. Plot the vapor-compression cycle in Ts coordinates (use NIST) and determine the cycle...
-
Refer to the quarterly financial report near the end of the notes to the financial statements in CVSs annual report. Is CVSs a seasonal business? Would you expect short-term borrowings and accounts...
-
As you learned in this chapter, XML allows users to define their own markup tags. You also learned that this flexibility can lead to problems when IT professionals who have developed tag sets for...
-
Describe the organizing framework for exporting. What steps should the firm follow to ensure exporting success? LO.1
-
The plaintiff , Betty Epstein, visited a beauty parlor to get her hair dyed. In the dying process, the beautician used a prebleach solution manufactured by Clairol, Inc., and then a commercial dye...
-
I vote a thumbs up! This is 1 question containing 3 requirements. Thank you! Lucido Products markets two computer games: Claimjumper and Makeover. A contribution format income statement for a recent...
-
Determine the product of the reaction: CH3 I CH3 -CH=C-CH3 + HBr CH3 T a) CHBr -CH=C-CH3 CH3 b) CH3 - CH -CH-CH3 CH3 c) CH3 -CH=CH-CH3 Br CH3 d) CH3 - CH -C-CH3 Br
-
Determine the products of each reaction. (a) CHCHCH=CH + Br (b) CHCHCH=CH + HBr
-
The arrangement of body axes of the fruit fly are shown in Figure 25.5g. Are the following statements true or false with regard to body axes in the mouse? A. Along the anteroposterior axis, the head...
-
1.A woman eats 65g of protein per day. She weighs 156 lbs. How much protein is she getting per kg of body weight? Explain briefly both question 2.152 lbs = ________________kg. [2.2 lbs = 1 kg] explain
-
The concrete slab shown in Figure is 7m x 5m. The slab is not supported along one of the 7m long edges (free edge). The other three edges are supported and continuous over the supports, and therefore...
-
1. If possible, find 4-B -[{3}][{3}] A=
-
Georgi owns 50% of Forbes, Inc., an S corporation. At the beginning of the current tax year, Georgi had zero basis and an unused net business loss carryover of $10,000. During the tax year, she...
-
luation, of Fundamental Managerial Accounting Concepts. Use Excelshowing all work and formulasto complete the following: Prepare a flexible budget. Compute the sales volume variance and the...
-
Drexel Sports Authority purchased inventory costing $23,000 by signing a 10%, six-month, short-term note payable. The purchase occurred on January 1, 2016. Drexel will pay the entire note (principal...
-
A Firm intends to invest some capital for a period of 15 years; the Firm's Management considers three Options, each consisting of purchasing a machinery of a specific brand, different for each...
-
Find the line charge density on a long wire if the electric field 45 cm from the wire has magnitude 260 kN/C and points toward the wire.
-
A molecule has its dipole moment aligned with a 1.2-kN/C electric field. If it takes 3.1*10 -27 J to reverse the molecules orientation, whats its dipole moment?
-
The electric field in a certain region is given by E(vector) = axi, where a = 40 N/Cm and x is in meters. Find the volume charge density in the region. (Apply Gausss law to a cube 1 m on a side.)
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


