Draw the Lewis structure for BrF with an arrow representing the dipole moment. Refer to Figure 10.10
Question:
Draw the Lewis structure for BrF with an arrow representing the dipole moment. Refer to Figure 10.10 to estimate the percent ionic character of the BrF bond.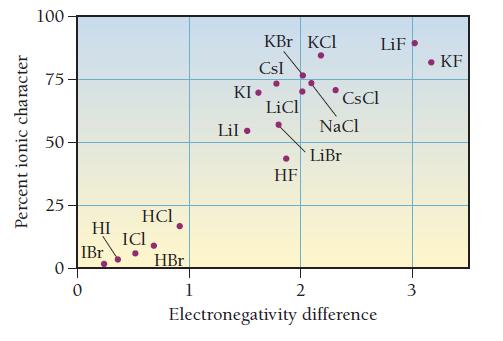
Transcribed Image Text:
Percent ionic character 100 75 50 25 0. HI IBr 0 HCI ICI. HBr 1 KBr KCI CsI KI. Lil. LiCl HF 2 CsCl NaCl LiBr LiF Electronegativity difference 3 • KF
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The Lewis structure for Brf with an arrow representing with the dipole moment and estimate the ionic ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the Lewis structure for CO with an arrow representing the dipole moment. Refer to Figure 10.10 to estimate the percent ionic character of the CO bond. Percent ionic character 100 75 50 25 0. HI...
-
The ionic character of the bond in a diatomic molecule can be estimated by the formula Where m is the experimentally measured dipole moment (in C m), e the electronic charge, and d the bond length in...
-
The Lewis structure for allene is Make a sketch of the structure of this molecule that is analogous to Figure 9.25. In addition, answer the following three questions: (a) Is the molecule planar? (b)...
-
How should employers that operate in different states and cities deal with lack of uniformity in employment laws?
-
What are database management systems? Are they the same as databases? Why are DBMSs classified as software and not hardware?
-
Wall parked his car in the parking lot at OHare Airport in Chicago. After he received a parking ticket from a ticket-dispensing machine, an automatic gate was raised and he entered the lot and parked...
-
Provide additional information about the salesvolume variance by calculating the sales-mix and sales-quantity variances
-
In 2010, Tarlo Company agrees to construct a highway for Brice County over a three-year period (2010 through 2012). The contract price is $1,200,000 and the construction costs (both actual and...
-
Ramer and Knox began a partnership by investing $78,000 and $117.000, respectively. During its first year, the partnership earned $230,000. Prepare calculations showing how the $230,000 Income is...
-
Powell's book warehouse distributes hardcover books to retail stores and extends credit terms of 2/10, n/30 to all of its customers. At the end of May, Powell's inventory consisted of books purchased...
-
Write the Lewis structure for each molecule or ion. a. H3COH b. OH- c. Bro- d. 0-
-
Determine if a bond between each pair of atoms would be pure covalent, polar covalent, or ionic. a. C and N b. N and S c. K and F d. N and N
-
The percentage of various colors are different for the peanut variety of M&MS candies, as reported on the Mars, Incorporated website: A 14-ounce bag of peanut M&MS is randomly selected and contains...
-
I think the waiter wrote in an extra \($25\) tip on my Sunshine Caf bill after I received and signed my credit card receipt, Mark Otter said to the restaurant manager, Brad Gladiolus. Mr. Otter, mail...
-
The following transactions occurred during January, the first month of operations for Red Corporation. Prepare journal entries and create a T-account for inventory that includes the following five...
-
Make T-accounts for the following accounts that appear in the general ledger of The Canine Hospital, owned by Kali Wells, a veterinarian: Cash; Accounts Receivable; Supplies; Office Equipment;...
-
On January 12, 2010, a major earthquake hit Haiti and the Dominican Republic. The epicenter of the quake was 25 kilometers southwest of the Haitian capital of Port-au-Prince and only 13 kilometers...
-
At the end of 2011, the following information is available for Short and Wise Companies: Required a. Set up the spreadsheet shown here. Complete the income statements by using Excel formulas. b....
-
A cylindrical specimen of a brass alloy having a length of 60 mm (2.36 in.) must elongate only 10.8 mm (0.425 in.) when a tensile load of 50,000 N (11,240 lbf) is applied. Under these circumstances,...
-
A bar of length = 1 has one fixed and one free end and stiffness function c(x) = 1 - x. Find the displacement when subjected to a unit force. Pay careful attention to the boundary condition at the...
-
What is the pressure, in psig, at the bottom of a swimming pool that is 10 ft deep?
-
If air has a constant specific weight of 0.075 lb/ft 3 , what pressure difference would result when driving from the base to the top of Pikes Peak, if the climb for the trip is 8400 ft?
-
Figure 4.21 shows a vacuum tank with a flat circular observation window in one end. If the pressure in the tank is 0.12 psia when the barometer reads 30.5 in of mercury, calculate the total force on...
-
Fredrickson Technologies last year paid a dividend of $2.4 per share. The company plans to keep these dividend payments fixed for the indefinite future. Assume that Fredrickson's equity cost of...
-
(Solving for n) How many years will it take for $520 to grow to $993.05 if it's invested at 6 percent compounded annually? Round Answer One Decimal Place
-
What would the planned shortage dollars be for the coat department if the planned net sales are $1,770,000 and the planned shortage percent is 1.9%

Study smarter with the SolutionInn App


