Find H rxn for the reaction: Use these reactions with known Hs: 3 C(s) + 4 H(g)
Question:
Find ΔHrxn for the reaction: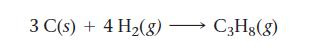
Use these reactions with known ΔH’s: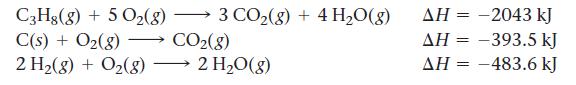
Transcribed Image Text:
3 C(s) + 4 H₂(g) → C3H8(8)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
To work this and other Hesss law problems manipulate the reactions with known AHs in such a way as t...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Can someone please summarize the case study below: if you know about the case IKEA Looks to Further Penetrate the U.S. Market 10 CASE Synopsis: IKEA is known around the world for its stylish,...
-
Allergic reactions to poison ivy can be miserable. Plant oils cause the reaction. Researchers at the Allergy Institute did a study to determine the effects of washing the oil off within 5 minutes of...
-
Coulometric titration of sulfite in wine. Sulfur dioxide is added to many foods as a preservative. In aqueous solution, the following species are in equilibrium: Bisulfite reacts with aldehydes in...
-
What is the "ALDI Way" and what was its quest? Take out costs; eliminate complexity Survival; make enough money to pay overhead costs Increase market share; find more prospective customers Build...
-
Fantastic Props, Inc., designs and fabricates movie props such as mock-ups of star-fighters and cybernetic robots. The companys balance sheet as of January 1, the beginning of the current year,...
-
Following is the stockholders' equity section from the Campbell Soup Company balance sheet. (Note: Campbell's uses shareowners' equity in lieu of the more common title of stockholders' equity.)...
-
Insert the missing phrase: Riskmanagement is not really a management fad. It provides a platform for . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . by giving comfort to shareholders...
-
Cheryl Sounders, the owner of Abruzzi, is currently developing a budget spreadsheet to explore the impact of various sales goals on production. In 2011, the company had monthly sales as follows:...
-
Question 4 of 10 ! 17 View Policies Current Attempt in Progress A $ 192 petty cash fund has cash of $ 41 and receipts of $174. The journal entry to replenish the account would include a O debit to...
-
The following table contains quarterly data on Upper Midwest car sales (CS) in thousands for 1996 Q1 through 2016 Q4: Upper Midwest Car Sales (CS) Year Q1 Q2 Q3 Q4 1996 407.6 431.5 441.6 306.2 1997...
-
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and in a coffee-cup calorimeter. In one of the calorimeters, q rxn = -12.5 kJ and in the other q rxn =...
-
Consider the reactions: What is H for the reaction 2 B 3 C? A 2 B A 3C ,
-
Show how to implement the FIFO queue ADT using only a priority queue and one additional integer instance variable.
-
5.Descibe the HSI color image model 6. Describe the basic relationship between the pixels
-
1. What is the need for transform? 2. What is Image Transform? 3. What are the applications of transform? 4. Give the Conditions for perfect transform . 5. What are the properties of unitary...
-
6. Define Fourier transform pair 7. Define Fourier spectrum and spectral density 8. Give the relation for 1-D discrete Fourier transform pair 9. Specify the properties of 2D Fourier transform. 10....
-
16. What is wrap around error? 17. Give the formula for correlation of 1D continuous function. 18. What are the properties of Haar transform. 19. What are the Properties of Slant transform 20....
-
21. Define fast Walsh transform. 22. Give the relation for 1-D DCT. 23. Write slant transform matrix SN. 24. Define Haar transform. 25. Define K-L transform. 26. Give the equation for singular value...
-
The permeability coefficient of a type of small gas molecule in a polymer is dependent on absolute temperature according to the following equation: where PM0and Qp are constants for a given...
-
Multiple Choice Questions: 1. The largest component of aggregate demand is? a. Government purchases. b. Net exports. c. Consumption. d. Investment. 2. A reduction in personal income taxes, other...
-
The strain at point A on the bracket has components ε x = 300 (10 -6 ), ε y = 550 (10 -6 ), γ xy = -650 (10 -6 ), ε z = 0. Determine (a) the...
-
The strain at point A on a beam has components ε x = 450(10 -6 ), ε y = 825(10 -6 ), γ xy = 275(10 -6 ), ε z = 0. Determine (a) the principal...
-
The strain at point A on the pressure-vessel wall has components ε x = 480(10 -6 ), ε y = 720(10 -6 ), γ xy = 650(10 -6 ). Determine (a) the principal strains at...
-
true- false statement (c) Cost-based accounting is conservative
-
C. Inventory Revaluation Outdoor Recreation has the following three trailers in stock at the end of the year: Model #1103 #1204 #1305 Original cost 5,500 7,200 9,000 Expected sales price 5,700 8,500...
-
true- false statement (8) Unanimity implies that shareholders have no incentive to use their voting rights. (1) With corporate income tax, retention dominates dividends

Study smarter with the SolutionInn App


