Given a barometric pressure of 762.4 mmHg, calculate the pressure of each gas sample as indicated by
Question:
Given a barometric pressure of 762.4 mmHg, calculate the pressure of each gas sample as indicated by the manometer.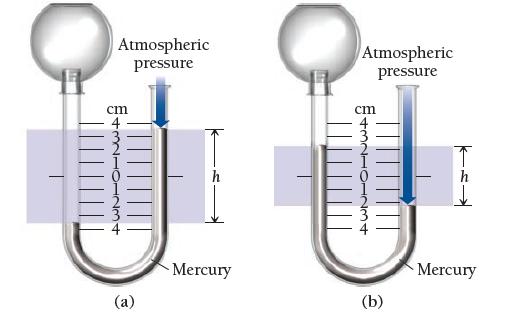
Transcribed Image Text:
Atmospheric pressure cm (a) Mercury Atmospheric pressure cm 11 (b) Mercury
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a 83...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Given a barometric pressure of 751.5 mmHg, calculate the pressure of each gas sample as indicated by the manometer. Atmospheric pressure cm (a) I Mercury Atmospheric pressure cm 4 MNOHY+ (b) Mercury
-
An 89.3 mL sample of wet O 2 (g) is collected over water at 21.3 C at a barometric pressure of 756 mmHg (vapor pressure of water at 21.3 C = 19 mmHg). (a) What is the partial pressure of O 2 (g) in...
-
(A) The reaction of aluminum with hydrochloric acid produces hydrogen gas. The balanced chemical equation for the reaction is given below. 2 Al(s) + 6 HCl(aq) 2 AlCl 3 (aq) + 3 H 2 (g) If 35.5 mL of...
-
How does the ERM system help with timely, effective, and democratic business decisions? A) Reporting to the presidents of each unit a faculty in a method that allows silo type decisions B) Board...
-
What is an enterprise system supposed to accomplish?
-
Which of the following describes the purpose of an intermediate timer event? a. Indicates receipt of a message b. Indicates branching c. Indicates delay d. Both (a) and (c) e. Both (b) and (c)
-
Terry Ray manages a hotel in an urban Midwestern city. He wants to use vertical analysis in order to evaluate his income statement (P&L). Complete the spreadsheet to help him evaluate his...
-
The net income of Steinbach & Sons, a department store, decreased sharply during 2014. Mort Steinbach, manager of the store, anticipates the need for a bank loan in 2015. Late in 2014, Steinbach...
-
K. Brant $800 $600$200 \ D. Eaton 500300 200\ S Klein 550\ 150 \ 400 \ At the beginning of the period, ABC had balances in Accounts Receivable of $100,000 and in Allowance for Doubtful Accounts of...
-
Determine the monthly cash flows and total cash generated at the end of each month and just before the payment is received for the construction of a house with the following budget and schedule. On...
-
A sample of gas has an initial volume of 5.6 L at a pressure of 735 mmHg. If the volume of the gas is increased to 9.4 L, what is its pressure?
-
The North American record for highest recorded barometric pressure is 31.85 in Hg, set in 1989 in Northway, Alaska. Convert this pressure to each indicated unit. a. MmHg b. Atm c. Torr d. KPa...
-
Consider the n-pentane (1) + methanol (2) system at 422.6 K in Figure P10-13. Four numbered black dots are provided on the Pxy diagram, and they are all at 422.6 K. For each of the four points,...
-
Watch Tre'Shawn's story (The QR code is in your text) https://www.youtube.com/watch?v=smIZLtDSPhU Using Chart 3.2 in your textbook describe what typical development for a 14-year-old boy would be...
-
Q17. An insurance company charges $500 for an insurance policy against fire and theft in the home. If a home is destroyed by fire, then the insurance company will pay the homeowner $250,000. What is...
-
If y = x ( 9 x + 5 ) , compute y ' .
-
1. Print out your name and section. 2. Create a java code to find speed of a car. a. Import the required codes to allow the user to enter data. b. The formula for speed is speed=distance/time. c. Ask...
-
Complete the square for 9 x 2 - 9 0 x + y 2 + 8 1 = 0
-
Why must rivets of a 2017 aluminum alloy be refrigerated before they are used?
-
The overall reaction and equilibrium constant value for a hydrogenoxygen fuel cell at 298 K is 2H 2 (g) + O 2 (g) 2H 2 O(l) K = 1.28 10 83 a. Calculate E cell and G 8 at 298 K for the fuel cell...
-
Determine the vertical displacement of joint D. Using Castiglianos theorem. AE is constant. Assume the members are pin connected at their ends. 000 4 m 4 m - 15 kN 20 kN 000
-
Determine the vertical displacement of joint D. Use the method of virtual work. AE is constant. Assume the members are pin connected at their ends. 000 4 m 4 m - 15 kN 20 kN 000
-
Determine the vertical displacement of joint E. For each member A = 400 mm 2 , E = 200 GPa. Using Castiglianos theorem. 1.5 m A JO 45 kN 2 m 2 m
-
For the buyer of 72 Jul Call, calculate the profit or loss (per contract) on the expiration date, when the spot price for CD is $0.7350
-
What if Timcos dividends grow at 6% for one year, than at 10% forever after that?
-
23. The employer's portion of payroll taxes amounted to $1,000 for social security, $400 for state unemployment, $200 for federal unemployment, and $400 for worker's compensation. Record the journal...

Study smarter with the SolutionInn App


