Given the data, calculate S vap for each of the first four liquids. (S vap = H
Question:
Given the data, calculate ΔSvap for each of the first four liquids.
(ΔSvap = ΔHvap/T, where T is in K)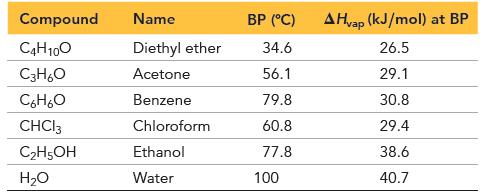
All four values should be close to each other. Predict whether the last two liquids in the table have ΔSvap in this same range. If not, predict whether it is larger or smaller and explain. Verify your prediction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





