Gold can be plated out of a solution containing Au 3+ according to the half-reaction: What mass
Question:
Gold can be plated out of a solution containing Au3+ according to the half-reaction:![]()
What mass of gold (in grams) is plated by a 25-minute flow of 5.5 A current?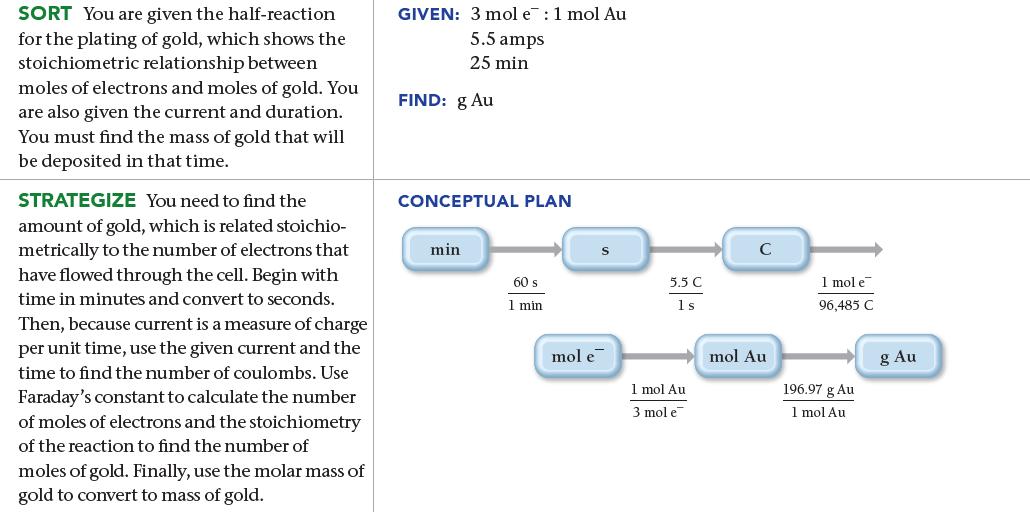
Transcribed Image Text:
3+ Au³+ (aq) + 3 e Au(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
25 min 60 X 1 min ...View the full answer

Answered By

Milan Mondal
I am milan mondal have done my Msc in physics (special astrophysics and relativity) from the University of burdwan and Bed in physical science from the same University.
From 2018 I am working as pgt physics teacher in kendriya vidyalaya no2 kharagpur ,west bengal. And also I am doing advanced physics expert in chegg.com .also I teach Bsc physics .
I love to teach physics and acience.
If you give me a chance I will give my best to you.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Aluminum can be plated out of a solution containing A l3+ according to the half-reaction: A l3+ (aq) +3e - Al (s) What mass of aluminum (in grams) will be plated by the flow of 50 A of current 10...
-
Silver nitrate solutions are often used to plate silver onto other metals. What is the maximum amount of silver (in grams) that can be plated out of 4.8 L of an AgNO 3 solution containing 3.4% Ag by...
-
A 50.0-mL portion of a solution containing 0.200 g of BaCl2 2H2O is mixed with 50.0 mL of a solution containing 0.300 g of NaIO3. Assume that the solubility of Ba(IO3)2 in water is negligibly small...
-
Write a Fortran program that creates an integer array with values -123, -4, 5, 67, 890, and 12345. Prints out the array in several formatted ways. a. Print each element, on its own line, using a...
-
Chemical Pro uses an automated mixing machine in its Mixing Department to combine three raw materials into a product called Triogo. On average, each unit of Triogo contains $3 of Material X, $6 of...
-
A patient visits her doctor with concerns about her blood pressure. If the systolic blood pressure exceeds 150, the patient is considered to have high blood pressure, and medication may be...
-
Identify and assess the main investment opportunities in Qatar for overseas investors? LO.1
-
Life insurance policies have different characteristics. For each of the following, identify the life insurance policy that meets the description: a. A policy where the face amount of insurance...
-
what am i doing wrong trying to find retained earnings? Check my work mode: This shows what is correct or incorrect for the work you have com requirea imrormauon [The following information applies to...
-
What is the definition of the standard cell potential (E cell )? What does a large positive standard cell potential imply about the spontaneity of the redox reaction occurring in the cell? What does...
-
Find E cell for an electrochemical cell based on the following reaction with [MnO 4 ] = 2.0 M, [H + ] = 1.0 M, and [Ag + ] = 0.010 M. Ecell for the reaction is +0.88 V. MnO4 (aq) + 4H+ (aq) + 3 Ag(s)...
-
Is increasing the entrepreneurial orientation of a firm always a good thing? Or are there circumstances or environments in which the further pursuit of opportunities can diminish firm performance?
-
From your reading this unit on motivation and change from the TIP series, what is the connection and interplay between these concepts/statements below in your opinion in working with clients facing...
-
Please help with the following The partnership of Bauer, Ohtani, and Souza has elected to cease all operations and liquidate its business property. A balance sheet drawn up at this time shows the...
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,200 cases of Oktoberfest-style beer from a German supplier for 276,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Define meaning of partnership deed.
-
List down the information contains in the partnership deed.
-
Moyle Co. acquired a machine on January I. Problem 6.26 2016, at a cost of $2.400.000. The machine is expected to have a five-year useful life. 1.0 3 with a salvage value of $150.000. The machine is...
-
Digital Fruit is financed solely by common stock and has outstanding 25 million shares with a market price of $10 a share. It now announces that it intends to issue $160 million of debt and to use...
-
Utilizing Eq. (11.38), show that F -1 {F{(x)}} = Æ(x). ik(x x') k 6( ') (11.38) 2
-
Given F{(x)}, show that F{(x - x 0 )} differs from it only by a linear phase factor.
-
Prove that * h = h * directly. Now do it using the convolution theorem.
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


