How many copper atoms are in a copper penny with a mass of 3.10 g? (Assume that
Question:
How many copper atoms are in a copper penny with a mass of 3.10 g? (Assume that the penny is composed of pure copper.)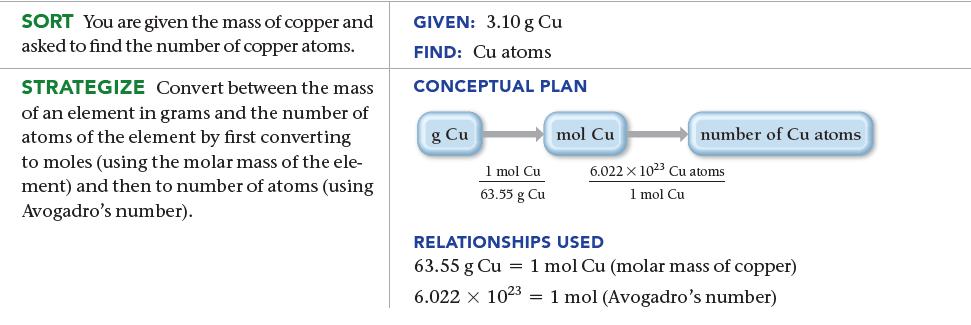
Transcribed Image Text:
SORT You are given the mass of copper and asked to find the number of copper atoms. STRATEGIZE Convert between the mass of an element in grams and the number of atoms of the element by first converting to moles (using the molar mass of the ele- ment) and then to number of atoms (using Avogadro's number). GIVEN: 3.10 g Cu FIND: Cu atoms CONCEPTUAL PLAN g Cu 1 mol Cu 63.55 g Cu mol Cu number of Cu atoms 6.022 x 1023 Cu atoms 1 mol Cu RELATIONSHIPS USED 63.55 g Cu = 1 mol Cu (molar mass of copper) 6.022 x 1023 1 mol (Avogadro's number)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
310 g x 1 mol ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Energy bands are considered continuous due to the large number of closely spaced energy levels. The range of energy levels in a crystal of copper is approximately 1 x 10 19 J. Assuming equal spacing...
-
Copper metal reacts with nitric acid. Assume that the reaction is 3Cu(s) + 8HNO3(aq) 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(l) If 6.01 g Cu(NO3)2 is eventually obtained, how many grams of nitrogen monoxide,...
-
Metallic copper adopts an fcc structure with density 8960 kg m 3 . Draw the unit cell of copper and mark the shortest copper atom to copper atom distance. How many copper atoms are there in the unit...
-
IKEA was founded in 1943 by a 17-year-old Swede named Ingvar Kamprad. The company, which initially sold pens, Christmas cards, and seeds from a shed on Kamprad?s family farm, eventually grew into a...
-
What do the terms target cost and target price mean? Explain how they are developed.
-
Why does sitting closest to the center of a vehicle provide the most comfortable ride in a bus traveling on a bumpy road, in a ship in a choppy sea, or in an airplane in turbulent air?
-
CEO: Its all well and good for you to say that I should disregard sunk costs when I consider whether to sell this unprofitable operation. After we report lower-than medium-size retailer of upscale...
-
The management of Zigby Manufacturing prepared the following estimated balance sheet for March, 2015: To prepare a master budget for April, May, and June of 2015, management gathers the following...
-
Nontaxable Fringe Benefits (LO. 4) Tia is married and is employed by Carrera Auto Parts. In 2020, Carrera established high-deductible health insurance for all its employees. The plan has a $2,800...
-
A bank decides to create a five-year principal-protected note on a non-dividend-paying stock by offering investors a zero-coupon bond plus a bull spread created from calls. The risk-free rate is 4%...
-
How and by whom was the electron discovered? What basic properties of the electron were reported with its discovery?
-
Determine the number of electrons in the Cr 3+ ion. a) 24 electrons b) 27 electrons c) 3 electrons d) 21 electrons
-
Shut down the computer and POS systems when you leave at night. When the computer system is on, the juice keeps flowingshutting it down can save significant energy bill dollars over the course of a...
-
Primare Corporation has provided the following data concerning last month's manufacturing operations. Purchases of raw materials Indirect materials used in production Direct labor Manufacturing...
-
3. Suppose we have n i.i.d., uniform-(0,t) random variables. Place these random variables on the interval (0, t]. Let 0 = 80 < 81 < ... < Sn1 < (0,t]. Skt. Compute the probability that there are in...
-
3. (3 pts) Use Python to write a function that takes a single input, a list of numbers. The function should loop through the list and, on each iteration, print the number if it is the largest number...
-
a) A linear charge density = 4z C/m is distributed on the z axis, what is the total charge within a cylinder of radius r = 0.5 m and height h = 5 m which extends from z = 1 to z = 4? b) A uniform...
-
Read the articles given below on module 9 now read the articles given below on module 10 Now answer these questions based on both modules slideshow pictures and the links readings Describe how the...
-
Here are traditional DNA fingerprints of five people: a child, mother, and three potential fathers: Which males can be ruled out as being the father? Explain your answer. If one of the males could be...
-
The population of Detroit, Michigan, decreased from 1,027,974 in 1990 to 688,701 in 2013 (Source: U.S. Census Bureau). Find the average rate of change in the population of Detroit, Michigan, over the...
-
A compound with molecular formula C 4 H 6 O 2 has the following NMR spectrum. Determine the number of protons giving rise to each signal. Proton NMR 25 2.0 2.0 1.5 ppm 3.0 Integration Values 5.0 4.5...
-
The 1 H NMR spectrum of a compound with molecular formula C 7 H 15 C l exhibits two signals with relative integration 2 : 3. Propose a structure for this compound.
-
For each of the following compounds, determine the multiplicity of each signal in the expected 1 H NMR spectrum: (a) (b) (c) (d)
-
please answer 2&3. Thanks! 2. The following information was taken from the financial records for Whitlock Industries for the years 2019 and 2020. The balance sheet items were recorded at the end of...
-
Alex and Bess have been in partnership for many years. The partners, who share profits and losses on a 60:40 basis, respectively, wish to retire and have agreed to liquidate the business. Liquidation...
-
Suppose a company estimates the following for a product it sells: Tangible value the product provides: $ 2 5 Intangible value the product provides: $ 1 5 Costs customer must incur to purchase the...

Study smarter with the SolutionInn App


