In order to determine the rate of photosynthesis (the conversion by plants of carbon dioxide and water
Question:
In order to determine the rate of photosynthesis (the conversion by plants of carbon dioxide and water into glucose and oxygen), the oxygen gas emitted by an aquatic plant is collected over water at a temperature of 293 K and a total pressure of 755.2 mmHg. Over a specific time period, a total of 1.02 L of gas is collected.
What mass of oxygen gas (in grams) forms?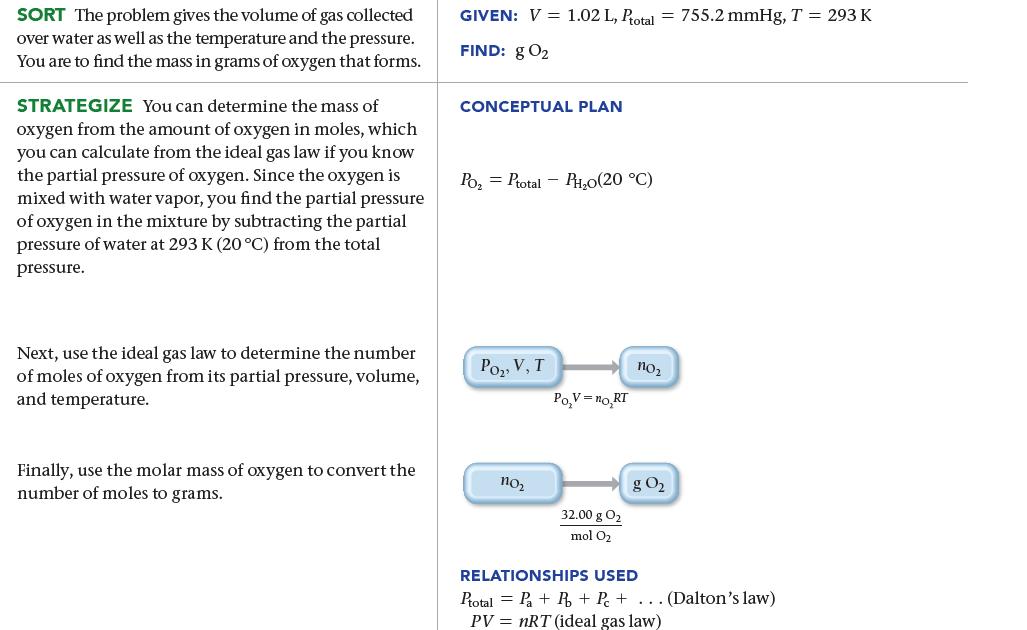
Transcribed Image Text:
SORT The problem gives the volume of gas collected over water as well as the temperature and the pressure. You are to find the mass in grams of oxygen that forms. STRATEGIZE You can determine the mass of oxygen from the amount of oxygen in moles, which you can calculate from the ideal gas law if you know the partial pressure of oxygen. Since the oxygen is mixed with water vapor, you find the partial pressure of oxygen in the mixture by subtracting the partial pressure of water at 293 K (20 °C) from the total pressure. Next, use the ideal gas law to determine the number of moles of oxygen from its partial pressure, volume, and temperature. Finally, use the molar mass of oxygen to convert the number of moles to grams. GIVEN: V = 1.02 L, Ptotal = 755.2 mmHg, T = 293 K FIND: g 0₂ CONCEPTUAL PLAN Po₂ Ptotal-PH₂0(20 °C) Po₂, V, T 110₂ Po,V=no,RT 32.00 g 0₂ mol O₂ no₂ g 0₂ RELATIONSHIPS USED Ptotal=Pa+ R + P +... (Dalton's law) PV = nRT (ideal gas law)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Poz 110 PtotalPHO20 C 7552 mmHg 1755 mmHg 7...View the full answer

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the cash flow diagram for the given project below and then calculate the project net profit. The following table shows the activities description, dependency, duration, and cost elements. Assume...
-
During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction: Suppose that a particular plant consumes 37.8 g of CO 2 in one week. Assuming...
-
A 1.604-g sample of methane (CH4) gas and 6.400 g of oxygen gas are sealed in a 2.50- L vessel at 411oC and are allowed to reach equilibrium. Methane can react with oxygen to form gaseous carbon...
-
2. Imagine a college basketball team has the following supply and demand figures for a typical game: Price 8 16 24 32 40 Quantity Demanded 28000 22000 16000 10000 4000 Quantity Supplied 16000 16000...
-
What is click fraud? Who benefits and who loses when click fraud occurs?
-
Which principle suggests the grouping up of highly cohesive classes? (a) REP (b) \(\mathrm{CCP}\) (c) ISP (d) OCP
-
Assume you own a restaurant that is successful in part because of a signature menu item with a secret recipe. Prepare a noncompete agreement for a chef you are hiring that you feel would be fair to...
-
Assume that you are auditing the inventory of Husky Manufacturing Company for the year ended December 31, 2013, and you are using MUS. The book value is $8,124,998.66. The risk of incorrect...
-
II.II On January 1, 2022, Chang Ltd. had $480,000 ordinary shares outstanding. During 2022, it had the following transactions that affected the ordinary share account. February 1 Issued 120,000...
-
DAT, Inc., needs to develop an aggregate plan for its product line. Relevant data are The forecast for next year is Management prefers to keep a constant workforce and production level, absorbing...
-
What is the ideal gas law? Why is it useful?
-
Calculate the root mean square velocity of I 2 ( g) at 373 K. a) 19.0 m/s b) 191 m/s c) 6.05 m/s d) 99.1 m/s
-
A turbine (Figure 5-7) has an efficiency of 80% and an outlet pressure of P = 0.5 bar. The inlet stream is at T = 300C, and can be either a saturated steam or a superheated steam at either P = 3 bar,...
-
Finding Confidence Intervals. In Exercises 9-16, assume that each sample is a simple random sample obtained from a population with a normal distribution. Professor Evaluation Scores Listed below are...
-
Pacifico Company, a U . S . - based importer of beer and wine, purchased 1 , 7 0 0 cases of Oktoberfest - style beer from a German supplier for 4 5 9 , 0 0 0 euros. Relevant U . S . dollar exchange...
-
7.C. a. When you add two vectors you get another vector: yes or no? b. When you subtract two vectors you get another vector: yes or no? c. Given the coordinate system below where increasing numbers...
-
Problem 1 At a given instant, the position of a plane at A and a train at B are measured relative to a radar antenna at O. Determine the distance d between A and B at this instant. To solve the...
-
The Bell-Boeing V-22 Osprey tiltrotor is both an airplane and a helicopter. It's advantage is the ability to rotate its engines and rotors to vertical position for take-off, landings, and helicopter...
-
Name the micro structural products of 4340 alloy steel specimens that are first completely transformed to austenite, then cooled to room temperature at the following rates: (a) 10C/s, (b) 1C/s, (c)...
-
How does Kant answer Humes bundle theory of self? Do you think he is successful?
-
Determine the moment at joints C and D, then draw the moment diagram for each member of the frame. Assume the supports at A and B are pins. EI is constant. 8 kN/m 6 m B 5 m
-
Determine the moment at joints A, B, C, and D, then draw the moment diagram for each member of the frame. Assume the supports at A and B are fixed. EI is constant. 3 m B 30 kN/m 3 m
-
Determine the moments at C and D, then draw the moment diagram for each member of the frame. Assume the supports at A and B are pins. EI is constant. 3k 12 ft D 6 ft A B -8 ft
-
0 Suppose that two different studies (A and B) have the same sample sizes in e four groups, with similar standard deviations in the four groups. Furthermore sample sizes and sample SDs are also the...
-
Please answer both, its the same question but a 2 part answer. Southwest Milling Company purchased a front-end loader to move stacks of lumber. The loader had a list price of $121,930. The seller...
-
Identify the at least two main ways to invest in real estate indirectly in your country. *MY COUNTRY IS UNITED STATES * Distinguish between direct and indirect investments in real estate.

Study smarter with the SolutionInn App


