Magnesium has three naturally occurring isotopes with the following masses and natural abundances: Sketch the mass spectrum
Question:
Magnesium has three naturally occurring isotopes with the following masses and natural abundances: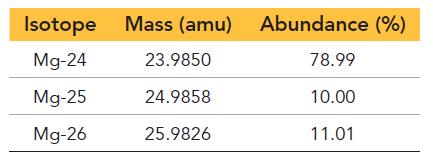
Sketch the mass spectrum of magnesium.
Transcribed Image Text:
Isotope Mg-24 Mg-25 Mg-26 Mass (amu) 23.9850 24.9858 25.9826 Abundance (%) 78.99 10.00 11.01
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
A mass spectrum is a graphical representation of the relative abundance of isotopes in ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Patients seeking care at the County General emergency room wait, on average, 6 minutes before seeing the triage nurse who spends, on average, 4 minutes assessing the severity of their problem. The...
-
An element has three naturally occurring isotopes with the following masses and abundances: Isotopic Mass (amu) Fractional Abundance 38.964 0.9326 39.964 1.000 104 40.962 0.0673 Calculate the atomic...
-
An element has three naturally occurring isotopes with the following masses and abundances: Isotopic Mass (amu) Fractional Abundance 27.977.. 0.9221 28.976.. 0.0470 29.974.. 0.0309 Calculate the...
-
Austin and Anya Gould are a middle-aged couple with two childrenRusty, age 13, and Sam, age 11whom they adopted this year. They also bought a new home in the area to give the children a yard in which...
-
Refer to the data for Balance, Inc., in Exercise 3-26. Required Using the Goal Seek function in Microsoft Excel, a. What number must Balance, Inc., sell to break even? b. What number must Balance,...
-
Boboli Co. wanted to promote its "California style" pizza, which it sold in supermarkets. The company contracted with Highland Group, Inc., to produce two million recipe brochures, which would be...
-
Define (1) information content and (2) the clientele effect, and explain how they affect dividend policy. AppendixLO1
-
Finned passages are frequently formed between parallel plates to enhance convection heat transfer in compact heat exchanger cores. An important application is in electronic equipment cooling, where...
-
Select all that apply, Stock Indexes: A. Weights can be proportional to market prices, capitalization B. Track changes in the value of a hypothetical portfolio of stocks C. Index calculation and...
-
Pizza Corporation acquired 80 percent ownership of Slice Products Company on January 1, 20X1, for $160,000. On that date, the fair value of the noncontrolling interest was $40,000, and Slice reported...
-
The atomic mass of fluorine is 18.998 amu, and its mass spectrum shows a large peak at this mass. The atomic mass of chlorine is 35.45 amu, yet the mass spectrum of chlorine does not show a peak at...
-
Which pair of elements do you expect to be most similar? Why? a. Nitrogen and oxygen b. Titanium and gallium c. Lithium and sodium d. Germanium and arsenic e. Argon and bromine
-
When an electric current is passed through water, hydrogen and oxygen gases are released. What STP volume of hydrogen gas is released from the electrolysis of 100.0 mL of water?
-
A closed square pyramid tank (base width: 6.0 m; height 3.0 m), sitting on its square base, has a 1.0 m depth of water. Suppose this tank is inverted (turned upside down) and is made to stand on its...
-
P.4.3 Apply a Taylor series expansion to a mixed backward formula for the first derivative: (Ux)i = 1 Ax (aui-2+ bui-1 + cu + dui+1) Derive the family of second order accurate formulas and the...
-
Show how you would go about balancing the following equations: Cu + HNO3 Cu(NO3)2 + NO + H2O HIO3 + Fel2 + HCI FeCl3 + ICI + H2O 2.Conservation of mass A student places 0.58 g of iron and 1.600 g...
-
Sales MOSS COMPANY Income Statement For Year Ended December 31, 2021 Cost of goods sold Gross profit Operating expenses (excluding depreciation) Depreciation expense Income before taxes Income taxes...
-
Prior to the Covid-19 epidemic, Master's and Ph.D. programs in psychology required applying students to submit their scores on the standardized graduate admission exam (GRE). For the past three...
-
Discuss the consequences of a germ-line versus a somatic mutation.
-
A city maintains a solid waste landfill that was 12 percent filled at the end of Year 1 and 26 percent filled at the end of Year 2. During those periods, the government estimated that total closure...
-
Using acetylene and 2-methylpropane as your only sources of carbon atoms, propose a plausible synthesis for 4-methyl-2-pentanone. You will need to utilize many reactions from previous chapters.
-
Draw a Lewis structure of a carbon atom that is missing one valence electron (and therefore bears a positive charge). Which second-row element does this carbon atom resemble in terms of the number of...
-
Below is the structure of caffeine, but its lone pairs are not shown. Identify the location of all lone pairs in this compound: - -N N. .C. Caffeine Z-O- Z-U
-
Albertini Co. purchased 33% of Lavender Corp's outstanding common stock as a long-term investment on Jan. 2, 2021. The following information relates to Lavender real estate assets. Book Value Fair...
-
Instructions The following transactions were completed by Irvine Company during the current fiscal year ended December 31: Feb. 8 May 27 Aug. 13 Oct 31 Received 40% of the $18,000 balance owed by...
-
Case 2 Mr.Fu has carried on his trading business in Hong Kong for many years. It is accepted that all his trading profits are sourced in Hong Kong. Mrs.Fu assists him to manage the business. He...

Study smarter with the SolutionInn App


