Making a Nonspontaneous Process Spontaneous. The hydrolysis of ATP, shown in Problem 91, is often used to
Question:
Making a Nonspontaneous Process Spontaneous. The hydrolysis of ATP, shown in Problem 91, is often used to drive nonspontaneous processes—such as muscle contraction and protein synthesis—in living organisms. The nonspontaneous process to be driven must be coupled to the ATP hydrolysis reaction. For example, suppose the nonspontaneous process is A + B → AB (ΔG° positive).
The coupling of a nonspontaneous reaction such as this one to the hydrolysis of ATP is often accomplished by the mechanism: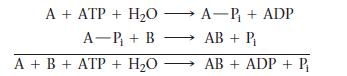
As long as ΔG°rxn for the nonspontaneous reaction is less than 30.5 kJ, the reaction can be made spontaneous by coupling in this way to the hydrolysis of ATP. Suppose that ATP is to drive the reaction between glutamate and ammonia to form glutamine: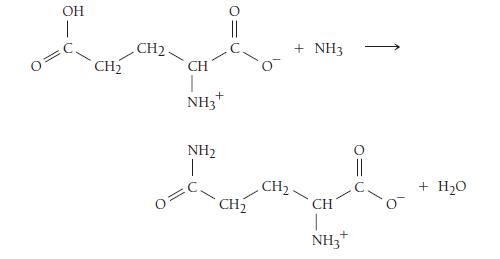
a. Calculate K for the reaction between glutamate and ammonia. (The standard free energy change for the reaction is +14.2 kJ/mol. Assume a temperature of 298 K.)
b. Write a set of reactions such as those given showing how the glutamate and ammonia reaction can couple with the hydrolysis of ATP. What are ΔG°rxn and K for the coupled reaction?
Problem 91
Living organisms use energy from the metabolism of food to create an energy-rich molecule called adenosine triphosphate (ATP). The ATP acts as an energy source for a variety of reactions that the living organism must carry out to survive. ATP provides energy through its hydrolysis, which can be symbolized as follows:![]()
where ADP represents adenosine diphosphate and Pi represents an inorganic phosphate group (such as HPO42–).
Step by Step Answer:






