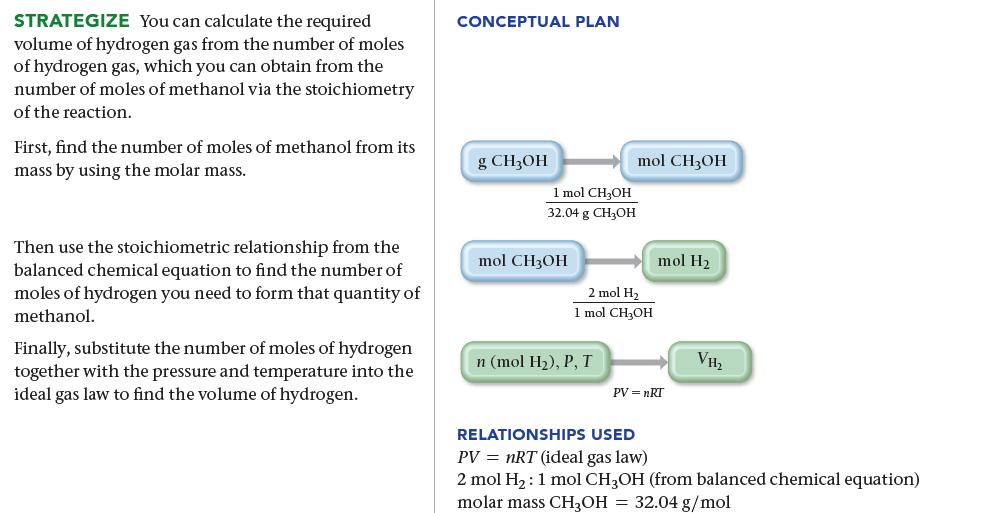
Methanol (CH 3 OH) can be synthesized by the reaction: What volume (in liters) of hydrogen gas,
Question:
Methanol (CH3OH) can be synthesized by the reaction:![]()
What volume (in liters) of hydrogen gas, at a temperature of 355 K and a pressure of 738 mmHg, is needed to synthesize 35.7 g of methanol?
Transcribed Image Text:
CO(g) + 2 H₂(8) CH3OH(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
357 g CH3OH X 11142 mol CH3OH X VH VH HRT P ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
CH 3 OH can be synthesized by the reaction: What volume of H 2 gas (in L), at 748 mmHg and 86 C, is required to synthesize 25.8 g CH 3 OH? How many liters of CO gas, measured under the same...
-
Methanol (CH 3 OH) can be synthesized by the reaction: CO(g) + 2 H 2 (g) CH 3 OH(g) What volume (in liters) of methanol gas, measured at a temperature of 473 K and a pressure of 820 mmHg, is...
-
Consider the chemical reaction: How many liters of hydrogen gas are formed from the complete reaction of 15.7 g C? Assume that the hydrogen gas is collected at a pressure of 1.0 atm and a temperature...
-
Question: If you were a consultant and for the below M&A case, what questions would you ask as a consultant of the acquiring company of the mine and in order to complete the valuation: A firm is...
-
What are Internet firewalls and proxy servers? How are they created? How do businesses use them for Internet security?
-
Which tasks are involved in the OOA process?
-
The most common operational business structures used in the hospitality industry.
-
At December 31, 2010, Appaloosa Corporation had a deferred tax liability of $25,000. At December 31, 2011, the deferred tax liability is $42,000. The corporations 2011 current tax expense is $48,000....
-
budgated at $2.70 per unit, and actual variable costs were $2.80 per unit, Actual fixed costs of $43,000 exceeded budgeted fixed costs by $4,600. Propare Stenback's fieciblo budget performance...
-
You should start and save a new Tableau file for each Chapter. Roger Company's files can be downloaded from Connect in Excel format (Click here). For Chapter 5, you will import the Roger Company...
-
Which gas has the greatest kinetic energy at STP? a) He b) Ne c) Ar d) None of the above (All have the same kinetic energy.)
-
What is the ideal gas law? Why is it useful?
-
"If supervisors communicate effectively with employees, deal with their concerns, and treat them fairly, employees are far less likely to be interested in forming or joining a union." Do you agree or...
-
"The initial speed with which a ball is thrown is doubled, with the angle of projection fixed. Is the maximum height to which the ball rises doubled?" Now, let's say you are also allowed to change...
-
Wally Working Co. emiti bonos con una tasa de inters nominal (contratada) de 15%, por un valor ominal de $80,000, con un vencimiento de 5 anios. Cuando emiti los bonos, la tasa de inters del mercado...
-
Using the Central Limit Theorem. In Exercises 5-8, assume that the amounts of weight that male college students gain during their freshman year are normally distributed with a mean of 1.2 kg and a...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
Please help Calculating NPV and IRR Businesses use NPV and IRR to determine whether a project will add - value for shareholders. After watching the CFA Level I Corporate Finance video, answer the...
-
On the basis of diffusion considerations, explain why fine pearlite forms for the moderate cooling of austenite through the eutectoid temperature, whereas coarse pearlite is the product for...
-
Quality Chicken grows and processes chickens. Each chicken is disassembled into five main parts. Information pertaining to production in July 2012 is: Joint cost of production in July 2012 was $50. A...
-
Determine the moment at B, then draw the moment diagram for each member of the frame. Assume the support at A is fixed and C is pinned. EI is constant. 2 kN/m A -3 m- 4 m
-
Determine the moments at Band D, then draw the moment diagram. Assume A and C are pinned and B and D are fixed connected. EI is constant. 8k -10 ft- 10 ft- -15 ft- 12 ft
-
Determine the moment that each member exerts on the joint at B, then draw the moment diagram for each member of the frame. Assume the support at A is fixed and C is a pin. EI is constant. 2 k/ft 6 ft...
-
Restate the following one-, three-, and six-month outright forward European term bid-ask quotes in forward points. Spot 1.3515 1.3532 One-Month 1.3528 1.3550 Three-Month 1.3544 1.3571...
-
The Storm Soccer Team sells season tickets and collects the cash in January at the beginning of the season. The team collected $57,750 for season tickets. The soccer season starts in February and the...
-
A company purchased $3,400 of merchandise on July 5 with terms 3/10, n/30. On July 7, it returned $600 worth of merchandise. On July 8, it paid the full amount due. The amount of the cash paid on...

Study smarter with the SolutionInn App


