Natural gas burns in air to form carbon dioxide and water, releasing heat. What minimum mass of
Question:
Natural gas burns in air to form carbon dioxide and water, releasing heat.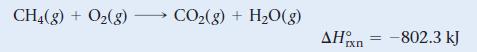
What minimum mass of CH4 is required to heat 55 g of water by 25 °C?
a) 0.115 g
b) 2.25 * 103 g
c) 115 g
d) 8.70 g
Transcribed Image Text:
CH4(g) + O2(g) CO2(g) + H2O(g) ΔΗ IX Π -802.3 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a...View the full answer

Answered By

Mercy Kangai
I provide creative and detailed administrative, web search, academic writing, data entry, Personal assistant, Content writing, Translation, Academic writing, editing and proofreading services. I excel at working under tight deadlines with strict expectations. I possess the self-discipline and time management skills necessary to have served as an academic writer for the past seven years. I can bring value to your business and help solve your administrative assistant issues. I have extensive experience in marketing and small business management.
4.80+
27+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
A gaseous hydrocarbon reacts completely with oxygen gas to form carbon dioxide and water vapor. Given the following data, determine Hof for the hydrocarbon: Hrxn = 2044.5 kJ/ mol Hof (CO2) = 393.5...
-
A 16.0-g sample of methane (CH 4 ) reacts with 64.0 g of oxygen gas in a container fitted with a piston (at 1.00 atm and 425 K). Methane can react with oxygen to form carbon dioxide and water vapor...
-
You are the Accounts Clerk for B2B Enterprises.You have been asked to complete the banking questions and bank reconciliation tasks below.To Do all tasks, the B2B EnterprisesOrganisationalPolicies and...
-
What arguments can be advanced in favor of treating fixed manufacturing overhead costs as period costs?
-
Is it possible for a business to differentiate its outputs and lower its costs simultaneously? Explain.
-
Suppose that Glass Creations computed its predetermined overhead rate on a quarterly basis instead of annually. The following projections have been made for the year: Estimated Estimated Quarterly...
-
Keswick Conference Center and Catering is a conference center and restaurant facility that hosts more than 300 national and international events each year attended by 50,000 professionals. Due to...
-
Required information The following information applies to the questions played below! Laker Company reported the following January purchases and sales data for its only product Date Activitin Accost...
-
Let x[n] = 0, n < 0, n > 7, be a real eight-point sequence, and let X[k] be its eight-point DFT. (a) Evaluate in terms of x[n]. (b) Let u[n] = 0, n < 0, n > 7, be an eight-point sequence, and let...
-
Explain how the sum of heat and work can be a state function, even though heat and work are themselves not state functions.
-
Which fuel is not a fossil fuel? a) Coal b) Hydrogen c) Natural gas d) Petroleum
-
The Hanks Company has prepared the following changes in account balances for the worksheet to support its 2007 statement of cash flows: Additional information: The net income was $1,300. Depreciation...
-
Think back to a time you experienced a communication breakdown in a personal or social setting (something you're comfortable discussing with the class in a public forum). 1. Did you figure out why...
-
Imagine you are visiting your aunt, who is a patient in a hospital in a nearby city. While you are sitting at her bedside, you hear a lot of noise at the nurses' station, as if they are having a...
-
Using Houseplan #5 on page 4 of the Measurement supplement(below), determine the cost of pouring the 9 inch thick concreteslab for this home, assuming that the porch will also be on thefoundation....
-
Recall from lecture that Flip-Flap Railway is an old roller coaster that was built in a circle. It has a diameter of 25 ft and riders entered the ride at a speed of 45 mph. At the top of the loop,...
-
Small Fry Design, founded in 1997, is a toy and accessories company that designs and imports products for children. The company's line of merchandise includes teddy bears, musical toys, rattles and...
-
Briefly explain how each of the following influences the tensile modulus of a semi crystalline polymer and why: (a) Molecular weight (b) Degree of crystallinity (c) Deformation by drawing (d)...
-
Refer to the table to answer the following questions. Year Nominal GDP (in billions) Total Federal Spending (in billions) Real GDP (in billions) Real Federal Spending (in billions) 2000 9,817 578...
-
Determine the bulk modulus for each of the following materials: (a) rubber, E r = 0.4 ksi, r = 0.48, and (b) glass, Eg = 8(10 3 ) ksi, g = 0.24.
-
The strain gage is placed on the surface of the steel boiler as shown. If it is 0.5 in. long, determine the pressure in the boiler when the gage elongates 0.2(10 -3 ) in. The boiler has a thickness...
-
The principal strains at a point on the aluminum fuselage of a jet aircraft are 1 = 780(10 -6 ) and 2 = 400(10 -6 ). Determine the associated principal stresses at the point in the same plane. E al...
-
Question Completion Status: QUESTION 7 When seven basketball players are about to have a free-throw competition, they often draw names out of a hat to randomly select the order in which they shoot....
-
Which is the best match for the situation described? Gap between espoused values and behavior, competing commitment, speaking the unspeakable, and work avoidence 1. A company has committed to...
-
An RCMP officer would like to estimate the true mean speed of all vehicles along a particular stretch of highway. She measures the speeds of a simple random sample of 50 vehicles and calculates a...

Study smarter with the SolutionInn App


