Nitric oxide reacts with chlorine gas according to the reaction: A reaction mixture initially contains equal partial
Question:
Nitric oxide reacts with chlorine gas according to the reaction: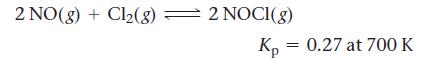
A reaction mixture initially contains equal partial pressures of NO and Cl2. At equilibrium, the partial pressure of NOCl is 115 torr. What were the initial partial pressures of NO and Cl2?
Transcribed Image Text:
2 NO(g) + Cl₂(8) = 2 NOCI(g) Kp 0.27 at 700 K =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
P NO...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Elemental phosphorus reacts with chlorine gas according to the equation: A reaction mixture initially contains 45.69 g P 4 and 131.3 g Cl 2 . Once the reaction has occurred as completely as possible,...
-
Pure nitrosyl chloride (NOCl) gas was heated to 240°C in a 1.00-L container. At equilibrium the total pressure was 1.00 atm and the NOCl pressure was 0.64 atm. (a) Calculate the partial pressures...
-
Aluminum reacts with chlorine gas according to the following equation shown below. How many moles of Cl 2 are required to react with 0.11 mol of Al? 2 Al( s ) + 3 Cl 2 ( g ) 2 AlCl 3 ( s )
-
Find the amplitude, period, and phase shift of function. Graph function. Show at least two periods. 2 3 - cos ( 6)
-
Stewart Fibers, Inc., specializes in the manufacture of synthetic fibers that the company uses in many products such as blankets, coats, and uniforms for police and firefighters. Stewart has been in...
-
Derive, for all three statistics, the relevant expressions for the quantity \(\left\langle n_{\varepsilon}^{2}ightangle-\left\langle n_{\varepsilon}ightangle^{2}\) from the respective probabilities...
-
Does the question contain gratuitous qualifiers? e.g. When you are driving your kids to school in the morning do you play music?
-
The manager of a department store in Seattle is attempting to decide on the types and amounts of advertising the store should use. He has invited representatives from the local radio station,...
-
A risk-free, zero-coupon bond with a face value of $10,000 has 20 years to maturity. If the YTM is 4.6%, which of the following would be closest to the price this bond will trade at? O A. $4,068 B....
-
A reaction vessel at 27 C contains a mixture of SO 2 (P = 3.00 atm) and O 2 (P = 1.00 atm). When a catalyst is added, this reaction takes place: 2 SO 2 (g) + O 2 (g) 2 SO 3 (g). At equilibrium, the...
-
The system described by the reaction CO(g) + Cl 2 (g) COCl 2 (g) is at equilibrium at a given temperature when PCO = 0.30 atm, PCl 2 = 0.10 atm, and PCOCl 2 = 0.60 atm. An additional pressure of Cl...
-
What are the principal advantages to a lessor in leasing rather than selling property?
-
Do you support the policy of not allowing some Chinese nationals to attend graduate school in the United States because of national security concerns?
-
Using your product or service name or category, do a search using the following phrase: Find a (insert the name of your product or service here...) near me. For instance, using my Mobile Notary...
-
Why do you think it is important to consider only relevant costs when conducting a differential analysis for a major purchase? Why not consider all possible costs in your decision? provide specific...
-
How do power dynamics and influence tactics shape decision-making processes and organizational politics within hierarchical structures ?
-
How do I answer these given the information below? Loan Amount? Loan to Value? Loan to Cost? Payment amount? Loan Balance at Maturity? Given Information: Property Cost: $1,000,000 Bank Policy on LTV:...
-
Lauren Lemaster works at the headquarters of a major Internet corporation that has offices in five countries. The company has a strict set of rules regarding the use of e-mail. Hackers often try to...
-
Why did management adopt the new plan even though it provides a smaller expected number of exposures than the original plan recommended by the original linear programming model?
-
For the data in Problem 10.22, compute the energy loss for gradual contractions with each of the cone angles listed in Figs. 10.10 and 10.11. Plot energy loss versus the cone angle. Figure 10.10...
-
Determine the energy loss for a gradual contraction from a 4-in Schedule 80 steel pipe to a 1-in Schedule 80 pipe for a flow rate of 250 gal/min. The cone angle for the contraction is 76.
-
Determine the energy loss for a sudden contraction from a 4-in Schedule 80 steel pipe to a 1-in Schedule 80 pipe for a flow rate of 250 gal/min.
-
Accountants should leverage technology such as artificial intelligence and robotic process automation (RPA) to A) Decrease the time spent on mundane tasks and increase the time spent on strategy...
-
Karen wants to have $27,471 in her investment account in 8 years. If her bank offers an annual compound interest rate of 2.6% with monthly compounding, how much should she deposit today?
-
In this task your supervisor has asked you to demonstrate and prove your understanding and ability to use the appropriate charts and tables to present and communicate findings of different categories...

Study smarter with the SolutionInn App


