One of these compounds is a liquid at room temperature. Which one and why? H-C-H Formaldehyde H
Question:
One of these compounds is a liquid at room temperature. Which one and why?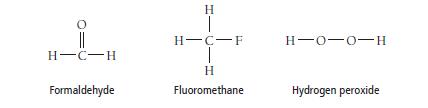
Transcribed Image Text:
H-C-H Formaldehyde H T H-C-F T H Fluoromethane H-0-0-H Hydrogen peroxide
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The three compounds have similar molar masses Formaldehyde 3003 gmol Fluoromethane 3403 ...View the full answer

Answered By

Sufiyan Ahmed Tariq
I am a Chartered Accountant and an Associate Public & Finance Accountant. I also hold a bachelors of Commerce degree. I have over 8 years of experience in accounting, finance and auditing. Through out my career, I have worked with many leading multinational organisation.
I have helped a number of students in studies by teaching them key concepts of subjects like accounting, finance, corporate law and auditing. I help students understanding the complex situation by providing them daily life examples.
I can help you in the following subject / areas:
a) Accounting;
b) Finance;
c) Commerce;
d) Auditing; and
e) Corporate Law.
4.90+
7+ Reviews
17+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
One of the following substances is a liquid at room temperature and the others are gaseous: CH 3 OH; C 3 H 8 ; N 2 ; N 2 O. Which do you think is the liquid? Explain.
-
Mercury is a liquid at room temperature and pressure, but its vapor is present in the atmosphere from natural sources, such as volcanoes, and from human ("anthropogenic") activities such as coal...
-
You are writing energy balances for a compound for which you cannot find heat capacity or latent heat data. All you know about the material are its molecular formula (C7H12N) and that it is a liquid...
-
Why would an organization use outplacement strategies before downsizing? Outplacing employees helps former employees develop new skill sets. Outplacement strategies are a means of eliminating problem...
-
Atwood Company has an opportunity to produce and sell a revolutionary new smoke detector for homes. To determine whether this would be a profitable venture, the company has gathered the following...
-
Holding other things constant, an appreciation of a nations currency causes a. exports to rise and imports to fall. b. exports to fall and imports to rise. c. both exports and imports to rise. d....
-
What, if anything, should be done to influence that? lop4
-
Server Farm Inc. (SFI) needs to upgrade its server computers. Company management has identified the following two options: (1) Shift to a Windows based platform from its current Unix-based platform,...
-
Question 11 2 pts In 2021, the dividends received deduction (DRD) for corporations owning less than 10% of a foreign corporation is: surse O 50% 65% 100% None of the above
-
You are interested in opening a new bakery in town. You are considering locations, and combinations of inputs to determine whether you should open, if you would be successful, and where you should...
-
This molecular diagram shows a sample of liquid water: Which of the diagrams below best depicts the vapor emitted from a pot of boiling water?
-
Explain why water drops are spherical in the absence of gravity.
-
The Ehrenfest urn model was originally proposed as a model for diffusion of gases, but has since come to be applied in a wide variety of fields. This problem is a simplified version of the model....
-
226 Payroll Accounting Chapter 7: Comprehensive Projects-Paper-Based Versions One-Month Project NOTE! Templates needed to complete these exercises, including one containing year-to-date payroll data,...
-
The Westchester Chamber of Commerce periodically sponsors public service seminars and programs Currently, promotional plans ore under way for this year's program Advertising alternatives include...
-
Mastery Problem: Activity-Based Costing WoolCorp WoolCorp buys sheep's wool from farmers. The company began operations in January of this year, and is making decisions on product offerings, pricing,...
-
The following system of linear equations is called underdetermined because there are more variables than equations. x2x 3x3 = 4 2x1x2 + 4x3 = -3 Similarly, the following system is overdetermined...
-
Write a 2000-word Reflection paper on " Country Managers Simulation by considering the following points: Countries chosen during the simulation were Argentina and Brazil: 1. Explain why you did what...
-
For each of the following abbreviated structural formulas, write a structural formula that shows all of the bonds: a. CH3(CH2)4CH3 b. (CH3)3CCH2CH2CH3 c. (CH3CH2)2NH d. CH3CH2SCH2CH3 e. ClCH2CH2OH f....
-
Differentiate. y = ln(3x + 1) ln(5x + 1)
-
The gear motor can develop 1/10 hp when it turns at 80 rev/min. If the allowable shear stress for the shaft is Ï allow = 4 ksi, determine the smallest diameter of the shaft to the nearest 1/8...
-
The gear motor can develop 1/10 hp when it turns at 300 rev/min. If the shaft has a diameter of 1/2 in., determine the maximum shear stress in the shaft.
-
The pump operates using the motor that has a power of 85 W. If the impeller at B is turning at 150 rev/min, determine the maximum shear stress in the 20-mm-diameter transmission shaft at A. 150...
-
For a company with the characteristics below, what would you expect the sustainable growth rate, g, to be? net income/share = $13.6 return on equity = 12.4% payout ratio = 39.9% plowback ratio =...
-
With an initial cost of $100,000, a WACC of 15%, and subsequent cash flows for years 1, 2, 3 of $25,000, $50,000, $75,000, in how many years will break even occur? Use non-discounted cash flows for...
-
Last month, Kaitlin's average daily balance on her credit card was $1,180.81. The annual interest rate on that credit card is 17.52%. The minimum payment on that card is the interest charge ( I...

Study smarter with the SolutionInn App


