One way to evaluate fuels with respect to global warming is to determine how much heat they
Question:
One way to evaluate fuels with respect to global warming is to determine how much heat they release during combustion relative to how much CO2 they produce. The greater the heat relative to the amount of CO2, the better the fuel. Use the combustion reactions of carbon, natural gas, and octane, in combination with the enthalpy of combustion for each reaction (all given earlier), to calculate the heat (in kJ) released by each fuel per 1.00 kg of CO2 produced.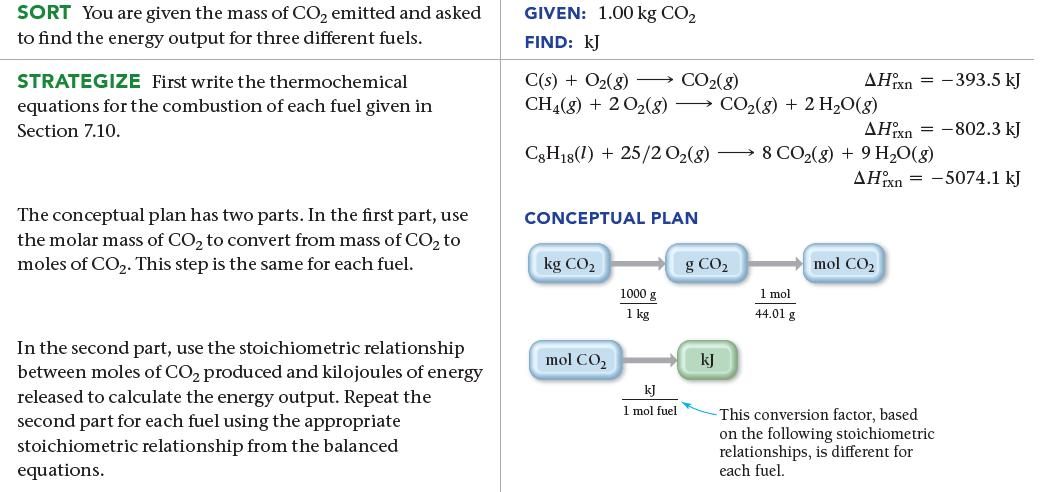

Transcribed Image Text:
SORT You are given the mass of CO₂ emitted and asked to find the energy output for three different fuels. STRATEGIZE First write the thermochemical equations for the combustion of each fuel given in Section 7.10. The conceptual plan has two parts. In the first part, use the molar mass of CO₂ to convert from mass of CO₂ to moles of CO₂. This step is the same for each fuel. In the second part, use the stoichiometric relationship between moles of CO₂ produced and kilojoules of energy released to calculate the energy output. Repeat the second part for each fuel using the appropriate stoichiometric relationship from the balanced equations. GIVEN: 1.00 kg CO₂ FIND: KJ C(s) + O₂(g) → CO₂(g) CH4(g) + 2O2(8) CO₂(g) + 2 H₂O(g) C8H18(1) + 25/2 02(8) → 8 CO₂(g) + 9 H₂O(g) CONCEPTUAL PLAN kg CO₂ mol CO₂ 1000 g 1 kg kJ 1 mol fuel g CO₂ kJ AHixn = 1 mol 44.01 g AHixn = -802.3 kJ -393.5 kJ AHixn=-5074.1 kJ mol CO₂ This conversion factor, based on the following stoichiometric relationships, is different for each fuel.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
100 kg CO X 1000 g 1 kg X For C 2272 mol C...View the full answer

Answered By

Lamya S
Highly creative, resourceful and dedicated High School Teacher with a good fluency in English (IELTS- 7.5 band scorer) and an excellent record of successful classroom presentations.
I have more than 2 years experience in tutoring students especially by using my note making strategies.
Especially adept at teaching methods of business functions and management through a positive, and flexible teaching style with the willingness to work beyond the call of duty.
Committed to ongoing professional development and spreading the knowledge within myself to the blooming ones to make them fly with a colorful wing of future.
I do always believe that more than being a teacher who teaches students subjects,...i rather want to be a teacher who wants to teach students how to love learning..
Subjects i handle :
Business studies
Management studies
Operations Management
Organisational Behaviour
Change Management
Research Methodology
Strategy Management
Economics
Human Resource Management
Performance Management
Training
International Business
Business Ethics
Business Communication
Things you can expect from me :
- A clear cut answer
- A detailed conceptual way of explanation
- Simplified answer form of complex topics
- Diagrams and examples filled answers
4.90+
46+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Multinational oil company ExxonMobil faced many challenges related to climate change. Climate change is taking place because of the greenhouse effect. When solar radiation passes through the...
-
In solar-heated buildings, energy is often stored as sensible heat in rocks, concrete, or water during the day for use at night. To minimize the storage space, it is desirable to use a material that...
-
Most businesses will incur debt at some point during their existence. Question 26 options: True False
-
Sharpton Fabricators Corporation manufactures a variety of parts for the automotive industry. The company uses a job-order costing system with a plantwide predetermined overhead rate based on direct...
-
What are the three motives for holding cash?
-
What was the culture at Lehman Brothers like? How did this culture contribute to the companys downfall?
-
The records of Reuben, Inc., reflect the following data: Work in process, beginning of month2,000 units one-half completed at a cost of $1,250 for materials, $675 for labor, and $950 for overhead....
-
Acompany will need 570.000 in a new son. To meet the pared, the company deporte money in un cert obey that may 10% rundt tervet compounded into Paderneta de mantent is a $70.000 ins The company who...
-
Table P-23 in Chapter 3 contains the quarterly income before extraordinary items for Southwest Airlines for the years 1988-1999. a. Using Minitab, smooth the Southwest Airlines income data using...
-
What is heat? Explain the difference between heat and temperature.
-
A city of 100,000 people uses approximately 1.0 * 10 11 kJ of energy per day. Suppose all of that energy comes from the combustion of liquid octane (C 8 H 18 ) to form gaseous water and gaseous...
-
Balance sheets and income statements for Target Corporation follow. \footnotetext{ Required a. Compute net operating profit after tax (NOPAT) for 2008 and 2007. Assume that the combined federal and...
-
Create your own privacy philosophy. This should cover the policies you will use for email, texting, social media, and internet usage. Consider what information is being collected anout you in each...
-
1. What future markets might be attractive to Carrefour and which mode of operation would be preferable? How important is the theoretical concept of psychological distance? 2. Corporate...
-
Briefly restate your problem space and methodology. Considering your problem space and methodology, what factors are you considering in deciding whether to use a theoretical foundation or a...
-
In light of your personal experience, what strategies or approaches do you believe could be effective in creating a workplace environment where employees from diverse cultural backgrounds feel both...
-
1. Entrepreneurs hold many common traits, identify five common traits of an entrepreneur that resonate with you and discuss each one of the five traits and why they matter to you. 2. Why is...
-
Make two schematic plots of the logarithm of relaxation modulus versus temperature for an amorphous polymer (curve C in Figure 15.8). (a) On one of these plots demonstrate how the behavior changes...
-
Tiger, Inc. signed a lease for equipment on July 1, 2007.The lease is for 10 years (the useful life of the asset).The first of 10 equal annual payments of $500,000 was made on July 1, 2007.The...
-
Initially, gaps between the A-36 steel plate and the rigid constraint are as shown. Determine the normal stresses Ï x and Ï y in the plate if the temperature is increased by T = 100°F....
-
The steel shaft has a radius of 15 mm. Determine the torque T in the shaft if the two strain gages, attached to the surface of the shaft, report strains of ε x² = -80(10 -6 ) and...
-
The shaft has a radius of 15 mm and is made of L2 tool steel. Determine the strains in the x² and y² direction if a torque T = 2 kN · m is applied to the shaft. 45 VT
-
Select one major economic indicator from either interest rates, unemployment, GDP, or inflation. What is the current level of this indicator and make a prediction for how this will move over the next...
-
What is the Profitability index (PI) of a project that has an initial cash outflow of $317,000 and the following cash inflows? Assume the required return is 12 percent. Year Cash inflow 1 $27,700 2...
-
business Law What is the legal name given to contractual arrangements between a debtor and its creditors for payment of debts that will allow a financially distressed business to continue operating?...

Study smarter with the SolutionInn App


