Recall that Cr and Cu are exceptions to the normal orbital filling, resulting in a [Ar] 4s
Question:
Recall that Cr and Cu are exceptions to the normal orbital filling, resulting in a [Ar] 4s13dx configuration.
Write the ground state electron configuration for each species.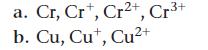
Transcribed Image Text:
Cr, Cr, Cr2+, Cr³+ a. b. Cu, Cut, Cu²+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a Ar4s3d ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Most of the second row transition metals do not follow the normal orbital filling pattern. Five of themNb, Mo, Ru, Rh, and Aghave a [Kr] 5s 1 4d x configuration and Pd has a [Kr] 4d 10 configuration....
-
When writing electron configurations, we use the building-up principle. However, there are exceptions to the building-up principle. An example is when a d-orbital will steal an electron from the...
-
Remaining time: 42:26 Question 1 What is the output of given code block? a = 15 b = 4 C = 3 print(a* b** c, c** 2, a % b - c, sep = "\\") Your answer: 960\\6\\0 960\6\15 960\9\15 180\9\0 960119110 O...
-
You are the senior auditor in charge of the December 31, 2018, year-end audit for Cleo Patrick Cosmetics Inc. (CPCI). CPCI is a large, privately held Canadian company that was founded in 1999 by one...
-
Nokia (www.nokia.com), the Finnish telecommunications company, has formed an equally owned joint venture with capital corporation, a state-owned Chinese company, to develop a center for the...
-
From the following table of prices per 100 pencils and quantities supplied (in hundreds of pencils), draw the supply curve for pencils: Price Quantity Supplied $5........... 20 $15............. 40...
-
The Food Faddist Price Index. A food faddist eats only steak, rice, and ice cream. In 1995, he bought: Item 1995 quantity 1995 price Steak 200 pounds $5.45/pound Rice 300 pounds 0.49/pound Ice cream...
-
1. This case study describes several different strategies for attracting and retaining new employees. On the basis of the four drives described in four drive theory and the needs listed in Maslows...
-
Compute trend percentages for Cedar Inc ' s net sales and net income for the following five - year period, using 2 0 1 9 as the base year K ( Click the icon to view net sales and net income for the...
-
Draw the Lewis diagrams for each ligand. Indicate the lone pair(s) that may be donated to the metal. Indicate any you expect to be bidentate or polydentate. a. CN b. Bipyridine (bipy), which has the...
-
Carbon monoxide and the cyanide ion are both toxic because they bind more strongly than oxygen to the iron in hemoglobin (Hb). Calculate the equilibrium constant value for this reaction: Does the...
-
Derive an expression for the internal pressure of a gas that obeys the Bethelot equation of state, P = RT/V m b a TV 2 m.
-
If Technical Specification 2 were reduced in the next design for this product, what would likely happen to customer opinion of Value Feature A? Quick Start QFD Matrix 2 Strong positive correlation...
-
Customer opinion of Value Feature B is most strongly correlated with what technical specification? Quick Start QFD Matrix 2 Strong positive correlation Some positive correlation == Strong negative...
-
Consider Quick Start QFD Matrix 1 above. Of the two value features, which do cus- tomers consider three times more important? Quick Start Quick Start QFD Matrix 1 = Strong positive correlation = Some...
-
Which technical spec can be most easily modified without changing current choices for the other two technical specs? Quick Start Quick Start QFD Matrix 1 = Strong positive correlation = Some positive...
-
Use Table A.1 to select 20 three-digit random numbers. Did any of the numbers occur more than once? How is it possible for a number to occur more than once? Make a stem-and-leaf plot of the numbers...
-
Several years ago, Cyclop Company issued bonds with a face value of $1,000,000 for $1,045,000. As a result of declining interest rates, the company has decided to call the bonds at a call premium of...
-
Evaluate the function at the given value(s) of the independent variable. Simplify the results. (x) = cos 2x (a) (0) (b) (- /4) (c) (/3) (d) ()
-
Two capacitors contain equal amounts of energy, yet one has twice the capacitance. How do their voltages compare?
-
Is a force needed to hold the plates of a charged capacitor in place? Explain.
-
Does the capacitance describe the maximum amount of charge a capacitor can hold, in the same way that a buckets capacity describes the maximum amount of water it can hold? Explain.
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


