Refer to Figure 12.36 to answer each question. a. A sample of steam begins on the line
Question:
Refer to Figure 12.36 to answer each question.
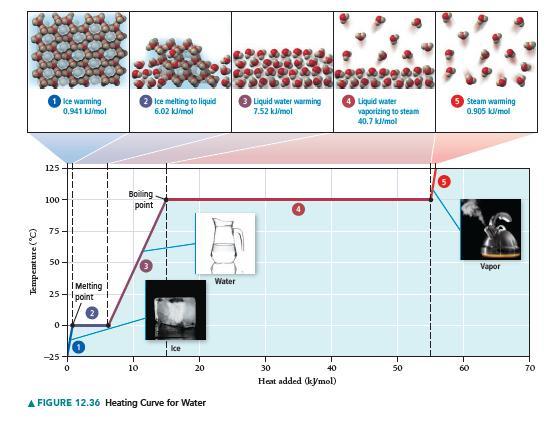
a. A sample of steam begins on the line segment labeled 5 on the graph. Is heat absorbed or released in moving from the line segment labeled 5 to the line segment labeled 3? What is the sign of q for this change?
b. In moving from left to right along the line segment labeled 2 on the graph, heat is absorbed, but the temperature remains constant. Where does the heat go?
c. How would the graph change if it were for another substance (other than water)?
Transcribed Image Text:
Temperature (°C) 125 100 Ice warming 0.941 kJ/mol 50- 1 I T 25-point 0. I -25- I 0 1 Melting! Ice melting to liquid 6.02 kJ/mol Bowling point 10 20 A FIGURE 12.36 Heating Curve for Water Water Liquid water warming 7.52 kJ/mol 30 40 Heat added (kymol) Liquid water vaporizing to steam 40.7 kJ/mol 50 5 Steam warming 0.905 kW/mol -8 Vapor 70
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
a The line segment labeled 5 on the graph represents the heating of steam vapor Moving from the line ...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
62+ Reviews
349+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Which of the below is the correct order of the consumption process? Question 2 options: Awareness; Thinking; Planning; Implementation/Action; Evaluation Evaluation; Thinking; Planning;...
-
Ralph Nader, who has been a critic of General Motors Corp. for several years, claims that when General Motors learned that Nader was about to publish a book entitled Unsafe at Any Speed, criticizing...
-
Liquidvapor equilibrium data have been collected for a binary system of methanol (1)water (2) at 40C. Mole fraction of liquid vs. total pressure are reported in the table below. Develop a computer...
-
What are missing data and why do they arise? Types of variable
-
Fighting Irish Incorporated pays its employees $5,600 every two weeks ($400/day). The current two-week pay period ends on December 28, 2015, and employees are paid $5,600. The next two-week pay...
-
Which of the following techniques is associated with an intrafirm comparison? A. None of the other choices are correct. B. Benchmarking a firm against another firm. C. Time series analysis. D....
-
Mrs. Yacumflastor, who is 68 years old, has correctly computed the following separate amounts in respect of income for tax purposes in 2023 for both her and her husband, who is 66 years old. (Amounts...
-
Suggest an explanation for the observation that the heat of fusion of a substance is always smaller than its heat of vaporization.
-
A root cellar is an underground chamber used to store fruits, vegetables, and even meats. In extreme cold, farmers put large vats of water into the root cellar to prevent the fruits and vegetables...
-
Select one of the following forum topics to research and write about. Week 2 Forum Topics - Chapter 2: Financial Statements, Cash Flow, and Taxes; Chapter 3: Analysis of Financial Statements 1. The...
-
Convert the following information into: a) a semantic net b) a frame-based representation A Ford is a type of car. Bob owns two cars. Bob parks his car at home.His house is in California, which is a...
-
Visit www.pearsonglobaleditions.com/malhotra to read the video case and view the accompanying video. Marriott: Marketing Research Leads to Expanded Offerings highlights Marriotts success in using...
-
The water level in a tank is \(20 \mathrm{~m}\) above the ground. A hose is connected to the bottom of the tank, and the nozzle at the end of the hose is pointed straight up. The tank cover is...
-
A simple experiment has long been used to demonstrate how negative pressure prevents water from being spilled out of an inverted glass. A glass that is fully filled by water and covered with a thin...
-
A golf ball is hit on a level fairway. When it lands, its velocity vector has rotated through an angle of 90. What was the launch angle of the golf ball? Pyo By Dyz =0 Uso Range R x max dya
-
Compare the intermediate benzenonium ions for ortho, meta and para bromination of benzoic acid, and explain why the main product is m-bromobenzoic acid. C-OH benzoic aciod
-
Pedro Bourbone is the founder and owner of a highly successful small business and, over the past several years, has accumulated a significant amount of personal wealth. His portfolio of stocks and...
-
Mutarotation causes the conversion of b-d-mannopyranose to -d-mannopyranose. Using Haworth projections, draw the equilibrium between the two pyranose forms and the open-chain form of d-mannose.
-
When d-talose is dissolved in water, an equilibrium is established in which two pyranose forms are present. Draw both pyranose forms and name them.
-
Draw the more stable chair conformation for each of the following compounds: (a) -d-Galactopyranose (b) -d-Glucopyranose (c) -d-Glucopyranose
-
A total of 2,000 units of Product A are produced from a joint process. Product A can be sold at the split-off point for $16 per unit, or it can be processed further for an additional total cost of...
-
How has COVID - 19 affected the job market in Canada Summary of all research conducted with a minimum of 3 credible sources? An activity you will be using to engage the audience and enforce the...
-
please as soon as can i will rate the thumps up IF THE BANK GIVES YOU 5% INTEREST ON YOUR SAVING ACCOUNT AND INFLATION IS 3% WHAT IS YOU Select one: O a. CANNOT BE CALCULATED b. 1.94% O c. 2% O d....

Study smarter with the SolutionInn App


