Referring to Table 18.1, pick an indicator for use in the titration of each base with a
Question:
Referring to Table 18.1, pick an indicator for use in the titration of each base with a strong acid.
a. CH3NH2
b. NaOH
c. C6H5NH2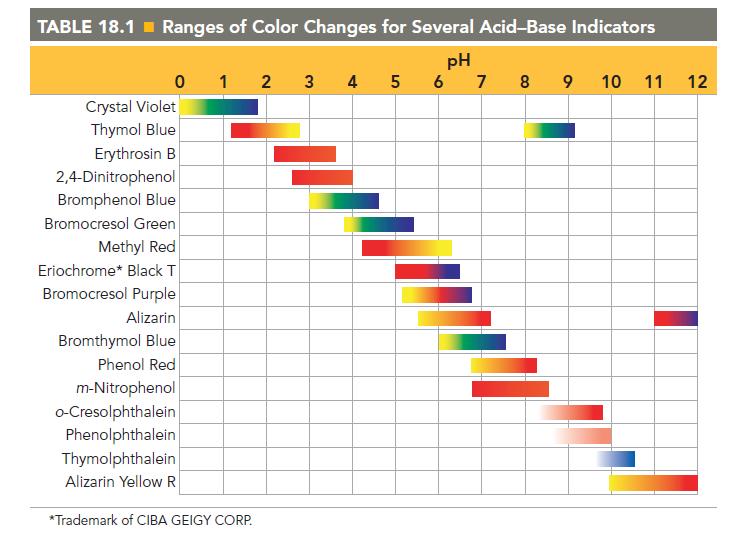
Transcribed Image Text:
TABLE 18.1 Ranges of Color Changes for Several Acid-Base Indicators pH 5 6 7 8 9 0 1 2 Crystal Violet Thymol Blue Erythrosin B 2,4-Dinitrophenol Bromphenol Blue Bromocresol Green Methyl Red Eriochrome* Black T Bromocresol Purple Alizarin Bromthymol Blue Phenol Red m-Nitrophenol o-Cresolphthalein Phenolphthalein Thymolphthalein Alizarin Yellow R *Trademark of CIBA GEIGY CORP. 3 4 tunnew 10 11 12
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Based on Table 181the following indicators are appropriate for use in the titration of each base wit...View the full answer

Answered By

Cristine kanyaa
I possess exceptional research and essay writing skills. I have successfully completed over 5000 projects and the responses are positively overwhelming . I have experience in handling Coursework, Session Long Papers, Manuscripts, Term papers, & Presentations among others. I have access to both physical and online library. this makes me a suitable candidate to tutor clients as I have adequate materials to carry out intensive research.
4.90+
1538+ Reviews
3254+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Referring to Table 18.1, pick an indicator for use in the titration of each acid with a strong base. a. HF b. HCl c. HCN TABLE 18.1 Ranges of Color Changes for Several Acid-Base Indicators pH 5 6 7 8...
-
Weak base B has a pK b of 6.78 and weak acid HA has a pK a of 5.12. a. Which is the stronger base, B or A ? b. Which is the stronger acid, HA or BH + ? c. Consider the following reaction: B(aq) +...
-
Consider the curve shown here for the titration of a weak base with a strong acid and answer each question. a. What is the pH and what is the volume of added acid at the equivalence point? b. At what...
-
On February 1, 2020, Sheridan Company sells merchandise on account to Carla Vista Company for $6490. The entry to record this transaction by Sheridan Company is Sales Revenue Accounts Payable Notes...
-
Sara Casper, a recent graduate of Rollings accounting program, evaluated the operating performance of Klumpe Companys six divisions. Sara made the following presentation to the Klumpe board of...
-
Write a brief paragraph describing the research project in Exercise 12.17 and its results.
-
2. $50,000 in cash to a local college for research purposes. $20,000 of the fund is immediately used to research a potential cure for cancer.
-
Suppose you know that a companys stock currently sells for $75 per share and the required return on the stock is 11 percent. You also know that the total return on the stock is evenly dividends...
-
On April 1, Jiro Nozomi created a new travel agency, Adventure Travel. The following transactions occurred during the company's first month. April 1 Nozomi invested $30,000 cash and computer...
-
Methyl red has a pK a of 5.0 and is red in its acid form and yellow in its basic form. If several drops of this indicator are placed in a 25.0-mL sample of 0.100 M HCl, what color will the solution...
-
A 20.0-mL sample of a 0.125 M diprotic acid (H 2 A) solution is titrated with 0.1019 M KOH. The acid ionization constants for the acid are K a1 = 5.2 * 10 -5 and K a2 = 3.4 * 10 -10 . At what added...
-
Yanmei Construction Company began operations on January 1, 2022. During the year, Yanmei Construction entered into a contract with Lundquist Corp. to construct a manufacturing facility. At that time,...
-
The following table contains the monthly operating costs of a company. Salary is not included. Determine the variance and standard deviation of the costs. Enero Febrero Marzo Abril Mayo Junio Julio...
-
Becker & Smith, CPAs, performs a financial statement review for BAM Markets ( BAM ) . Caroline, the manager on the job, learns that Don, a member of the review team, violated the independence rules....
-
Presented here are selected transactions for Sheridan Inc. during August of the current year. Sheridan uses a perpetual inventory system. It estimates a return rate of 10%, based on past experience....
-
. Complete both parts (a) and (b) below. ). In1 (a) Let X11, X12, ..., X be a random sample of size n from a population with mean and variance . Let X21, X22,..., X2n2 be a random sample of size n...
-
41. Let S be the cone z = x + y, z 2, oriented with outward unit normal. Use Stokes' theorem to evaluate the flux integral for the vector field SJ (V x F). ndS F(x, y, z) = (x y)i + 2zj + xk. -
-
Information for Schopp Corporation is given in E8-5. In E8-5 Schopp Corporation makes a mechanical stuffed alligator that sings the Martian national anthem. The following information is available for...
-
A spacecraft has left the earth and is moving toward Mars. An observer on the earth finds that, relative to measurements made when the spacecraft was at rest, its a. length is shorter b. KE is less...
-
From which class of solids would you expect electrons to be liberated most readily by the photoelectric effect when light is shined on a sample?
-
You are given two solids that look nearly alike, one of which is held together by ionic bonds and the other by van der Waals forces. How could you tell them apart?
-
What kind of solid is ice? Why does ice float when nearly all other solids sink when they freeze?
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


