Referring to Table 25.2, explain why you might expect Cr and Fe to form miscible alloys. TABLE
Question:
Referring to Table 25.2, explain why you might expect Cr and Fe to form miscible alloys.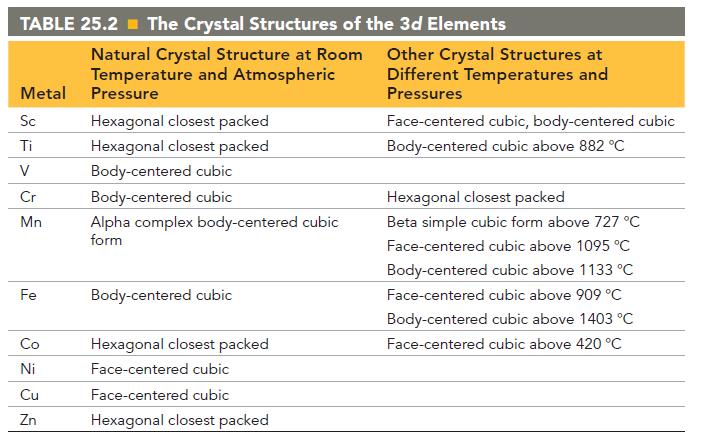
Transcribed Image Text:
TABLE 25.2 The Crystal Structures of the 3d Elements Metal Sc Ti V Cr Mn Fe Co 8 235 Ni Cu Zn Natural Crystal Structure at Room Temperature and Atmospheric Pressure Hexagonal closest packed Hexagonal closest packed Body-centered cubic Body-centered cubic Alpha complex body-centered cubic form Body-centered cubic Hexagonal closest packed Face-centered cubic Face-centered cubic Hexagonal closest packed Other Crystal Structures at Different Temperatures and Pressures Face-centered cubic, body-centered cubic Body-centered cubic above 882 °C Hexagonal closest packed Beta simple cubic form above 727 °C Face-centered cubic above 1095 °C Body-centered cubic above 1133 °C Face-centered cubic above 909 °C Body-centered cubic above 1403 °C Face-centered cubic above 420 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Cr and Fe are very close to each o...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Referring to Table 25.2, explain why you might expect Co and Cu to not form miscible alloys. TABLE 25.2 The Crystal Structures of the 3d Elements Metal Sc Ti V Cr Mn Fe Co 8 235 Ni Cu Zn Natural...
-
Explain why you might expect the MSE to be smaller in Question 2 than in Question 1.
-
Explain why you might expect stocks to have nonzero alphas if the market proxy portfolio is not highly correlated with the true market portfolio, even if the true market portfolio is efficient?
-
Write the formulas of these compounds: sulfur trioxide; phosphorus pentachloride; dinitrogen tetroxide.
-
Romans Construction Company purchased a new crane for $721,000 at the beginning of year 1. The crane has an estimated residual value of $70,000 and an estimated useful life of six years. The crane is...
-
Eagle Gate College hired an admission consultant from Stevens-Henager College (Janna Miller). Ms. Miller hired others from Stevens-Henager to join her at Eagle Gate. Those who came along to join Ms....
-
A transport company is running two buses between two places 100 km apart. Seating capacity of each bus is 50 passengers. The following particulars are taken from their books for a month. Rs Wages of...
-
Hanna Railroad Co. is about to issue $300,000 of 10-year bonds paying a 9% interest rate, with interest payable semiannually. The discount rate for such securities is 8%. How much can Hanna expect to...
-
After graduating from Hult International Business School with honors, you started working for a global investment bank as a stock analyst. Congratulations! You are working on your very first stock...
-
Determine the composition of each cobalt alloy. a. One-third of the Co atoms are replaced by Zn atoms. b. One-eighth of the Co atoms are replaced by Ti atoms. c. One-third of the Co atoms are...
-
Determine the composition of each vanadium alloy. a. One-half of the V atoms are replaced by Cr atoms. b. One-fourth of the V atoms are replaced by Fe atoms. c. One-fourth of the V atoms are replaced...
-
What is the relationship between political freedom and economic freedom? LO.1
-
Give your overall opinion . What do you think about neuromarketing? Is it usefull or is it a waste of time? Some people think this practice is "Orwellian", do you agree? Can marketers manage the...
-
Question 1. Let z= f(x,y), x = g (s, t). and ' y = h (s, t). with f, g & h all differentiable. (a) Set up an appropriate tree diagram for the of chain rule as done in this module's Use video lessons:...
-
ow do synergistic dynamics emerge within high-performance teams, and what role do diverse skill sets, complementary roles, and shared goals play in fostering collaborative innovation and collective...
-
(14%) Problem 3: The circuit shown contains a voltage source with emf & = 5.99 V, a resistor with resistance R = 135 k2, and a capacitor with capacitance C = 507 nF. When switch S is set to position...
-
1. What functions do all managers perform regularly? How do these functions apply to the three levels of management found in most organizations? 2. Identify and distinguish between the different...
-
Assume that you are the president of Influence Corporation. At the end of the first year (December 31) of operations, the following financial data for the company are available: Cash...
-
Evaluate how many lines there are in a true rotational spectrum of CO molecules whose natural vibration frequency is w = 4.09 1014 s1 and moment of inertia I = 1.44 1039 g cm2.
-
A current of 0.75 A fl ows through a lightbulb for 1 h. How many electrons pass through the lightbulb in this time?
-
Electrons move between points A and B in Figure P19.4 at a rate of 15 electrons per second. What is the current? Give the magnitude and direction of I. -e re Figure P19.4
-
During a thunderstorm, a lightning bolt carries current between a cloud and the ground below. If a particular bolt carries a total charge of 20 C in 1.0 ms, what is the magnitude of the current? How...
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


