Referring to the tables in Appendix IIB, determine if dinitrogen monoxide is stable at room temperature compared
Question:
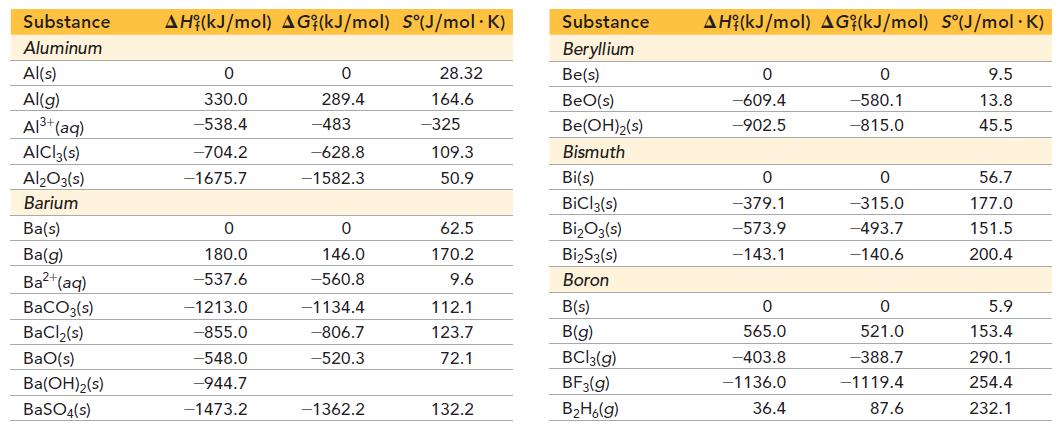
Referring to the tables in Appendix IIB, determine if dinitrogen monoxide is stable at room temperature compared to its elements, O2 and N2. Is dinitrogen monoxide stable at any temperature?
Appendix IIB
Transcribed Image Text:
Substance Aluminum Al(s) Al(g) Al³+ (aq) AICI3(s) Al₂O3(s) Barium Ba(s) Ba(g) Ba²+ (aq) BaCO3(s) BaCl₂(s) BaO(s) Ba(OH)2(s) BaSO4(s) AH (kJ/mol) AG (kJ/mol) S(J/mol. K) 0 330.0 -538.4 -704.2 -1675.7 0 180.0 -537.6 -1213.0 -855.0 -548.0 -944.7 -1473.2 0 289.4 -483 -628.8 -1582.3 0 146.0 -560.8 -1134.4 -806.7 -520.3 -1362.2 28.32 164.6 -325 109.3 50.9 62.5 170.2 9.6 112.1 123.7 72.1 132.2 Substance Beryllium Be(s) BeO(s) Be(OH)₂(s) Bismuth Bi(s) BiCl3(s) Bi₂O3(s) Bi₂S3(s) Boron B(s) B(g) BC13(g) BF3(g) B₂H6(g) AH (kJ/mol) AG (kJ/mol) S°(J/mol.K) 0 -609.4 -902.5 0 -379.1 -573.9 -143.1 0 565.0 -403.8 -1136.0 36.4 0 -580.1 -815.0 0 -315.0 -493.7 -140.6 0 521.0 -388.7 -1119.4 87.6 9.5 13.8 45.5 56.7 177.0 151.5 200.4 5.9 153.4 290.1 254.4 232.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Referring to the tables in Appendix IIB dinitrogen monoxide N2O is not stable at room temperature co...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Referring to the tables in Appendix IIB, determine whether or not hydrogen azide is stable at room temperature compared to its elements, H 2 and N 2 . Is hydrogen azide stable at any temperature?
-
5 kg of refrigerant 134a at a pressure of 7.5 bar and 0.7 quality is to be cooled inside a sealed rigid container until the pressure reaches 2.1 bar. Referring to the tables in Appendix A.4,...
-
Triphenylmethyl radical, (C 6 H 5 ) 3 C, is stable at room temperature in dilute solution in an inert solvent, and salts of triphenylmethyl cation, (C 6 H 5 ) 3 C + , can be isolated as stable...
-
Taylors 2022 health insurance premiums of $7,800 are paid by her employer. During 2022, Taylor requires surgery on her vocal chords. The cost of the surgery is $10,000 and Taylors insurance covers...
-
Dalston Lui, the accountant at Brightlight, Inc., Must group the costs of manufacturing candles. Indicate whether each of the following items should be classified as direct materials (DM), direct...
-
The following scenarios describe the price elasticity of supply and demand for a particular good. In which scenario will a subsidy increase consumption the most? Choose only one. a. Elastic demand,...
-
A factory producing an article P also produces a by-product Q, which is further processed into finished product. The joint cost of manufacture is as follows: Rs Materials 5,000 Labour 3,000 Overheads...
-
At the end of Stampfer Department Store's fiscal year on November 30, 2014, these accounts appeared in its adjusted trial balance. Freight-In ................................................... $...
-
In each of the following transactions (a) through ( for Catena's Marketing Company, indicate the amounts and the direction of effects of the adjusting entry on the elements of the balance sheet and...
-
Describe how red and black phosphorus are made from white phosphorus.
-
Describe the differences in the allotropes of white and red phosphorus. Explain why red phosphorus is more stable.
-
Street Smarts, Inc., mass-produces street lights. Materials used in constructing the body of the lights are added at the start of the process, while the materials used in wiring the lights are added...
-
Sample for a Poll There are 30,488,983 Californians aged 18 or older. If The Gallup organization randomly selects 1068 adults without replacement, are the selections independent or dependent? If the...
-
Part A: You have successfully graduated Conestoga College and have joined a public accounting firm in their tax department. You have been assigned to work on a project with Emily Wilson, one of the...
-
Write a program that gets a list of integers from input, and outputs negative integers in descending order (highest to lowest). Ex: If the input is: 10 -7 4-39 -6 12 -2 the output is: -2-6-7-39 For...
-
The manager of a division that produces add-on products for the automobile industry had just been presented the opportunity to invest in two independent projects. The first is an air conditioner for...
-
4. We are interested in the effect on test scores of the student-teacher ratio (STR). The following regression results have been obtained using the California data set. All the regressions used...
-
A company uses the indirect method to prepare the statement of cash flows. Indicate whether each of the following transactions affects an operating activity, an investing activity, a financing...
-
5. Convert the following ERD to a relational model. SEATING RTABLE Seating ID Nbr of Guests Start TimeDate End TimeDate RTable Nbr RTable Nbr of Seats RTable Rating Uses EMPLOYEE Employee ID Emp...
-
Consider two inductors L 1 and L 2 connected in parallel as shown in Figure P21.45. These two inductors act as one equivalent inductance L equiv . To find L equiv , we first notice that because they...
-
What is the energy stored in the inductor in Figure P21.46 after the switch has been closed for a very long time? R = 1000 N V = 3.0 V L = 5.0 mH Figure P21.46
-
Consider an MRI (magnetic resonance imaging) magnet that produces a magnetic field B = 1.5 T at a current I = 140 A. Assume the magnet is a solenoid with a radius of 0.30 m and a length of 2.0 m. (a)...
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...
-
Your portfolio has a beta of 1.17, a standard deviation of 14.3 percent, and an expected return of 12.5 percent. The market return is 11.3 percent and the risk-free rate is 3.1 percent. What is the...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...

Study smarter with the SolutionInn App


