Silver sulfate dissolves in water according to the reaction: A 1.5-L solution contains 6.55 g of dissolved
Question:
Silver sulfate dissolves in water according to the reaction: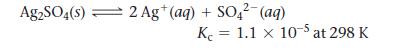
A 1.5-L solution contains 6.55 g of dissolved silver sulfate. If additional solid silver sulfate is added to the solution, will it dissolve?
Transcribed Image Text:
Ag2SO4(s) 2 Ag+ (aq) + SO42- (aq) K 1.1 x 10-5 at 298 K =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
Additi...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
A sample consisting of 22.7 g of a nongaseous, unstable compound X is placed inside a metal cylinder with a radius of 8.00 cm, and a piston is carefully placed on the surface of the compound so that,...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
To the right is the graph of the position of the object versus time: Which of graphs below correctly shows the object's velocity versus time? velocity (m/s) velocity (m/s) 1.5 1 0.5 -0.5 -1 -1.5 15...
-
Jonfran Company manufactures three different models of paper shredders including the waste container, which serves as the base. While the shredder heads are different for all three models, the waste...
-
Given the lending returns presented in Table 2.13 compute the average and variance of the returns on the banks portfolio. What is the probability that the interest rate on the banks loan portfolio is...
-
Explain some of the major difficulties involved when analyzing financial statements pro vided by companies from foreign countries.
-
Beachfront property owners of the Town of Eden Beach requested a seawall be constructed to protect their beach. The seawall was financed through a note payable, which was to be repaid from taxes...
-
Question 5 of 6 < View Policies Current Attempt in Progress -/1 E Crane Corbin's regular hourly wage rate is $14, and she receives an hourly rate of $21 for work in excess of 40 hours. During a...
-
Part of Glo's strategic plan is to capture market segments that are traditionally underserved. Glo's management believes that the market for men's care products is underserved and would be an...
-
Nitrogen dioxide dimerizes according to the reaction: A 2.25-L container contains 0.055 mol of NO 2 and 0.082 mol of N 2 O 4 at 298 K. Is the reaction at equilibrium? If not, in what direction will...
-
Consider the reaction: A reaction mixture contains 0.112 atm of H 2 , 0.055 atm of S 2 , and 0.445 atm of H 2 S. Is the reaction mixture at equilibrium? If not, in what direction will the reaction...
-
In May 2008, Jasper Mason died, survived by his spouse Amber Mason and four adult children. His gross estate was valued at $3 million, and he had Sec. 2053 deductions of $120,000. His will left the...
-
The formula weight (FW) of a gas can be determined using the following form of the ideal gas law FW = g R T / PV where g is the mass in grams, R is the gas constant, T is the temperature in Kelvin, P...
-
Consider a game in which a fair die is thrown. The player pays $5 to play and wins $2 for each dot that appears on the roll. Define X = number on which the die lands, and Y = player's net profit...
-
2. Getting ready for Logarithms and Calculus! a. Fill in the chart and graph the function (I advise practicing on your scientific calculator and desmos. X f(x) = Inx 0 0.5 1 e 10...
-
JoJo Co. had the following balances and information for October. Beg. finished goods inventory = $30 Beg. work in process inventory = $5 Beg. raw materials inventory = $15 End. finished goods...
-
Subway sales have been declining since 2014. In the US, Subway has closed a number of stores due to over-expansion, outdated operations, and uninspiring menus. In Canada, Subway took a different...
-
Consider the Vasicek Model under risk neutral probability measure: drt = ( rt)dt + dWt (16.3) with r0 = 0.01, = 0.2, = 0.01, = 0.05. Write a Matlab program to simulate a sample path of rt from t...
-
A spacecraft has left the earth and is moving toward Mars. An observer on the earth finds that, relative to measurements made when the spacecraft was at rest, its a. length is shorter b. KE is less...
-
A bartender slides a mug of root beer with mass m = 2.6 kg down a bar top of length L = 2.0 m to an inattentive patron who lets the mug fall a height h = 1.1 m to the floor. The bar top (Fig. P4.72)...
-
Consider once again the swimmer in Example 4.7. Assume she can swim at a velocity of 0.30 m/s and the river is 15 m wide. She needs to get across the river as quickly as possible. (a) What direction...
-
You are a serious basketball player and want to use physics to improve your free-throw shooting. Do an approximate calculation of the minimum speed the ball must have to travel from your hand to the...
-
For a firm with the following characteristics, what is it's book value? Current assets = 8,000 Fixed assets = 10,000 Total assets = 18,000 Current liabilities = 5,000 Long term liabilities = 9,000...
-
Sonia wants to have $11,000 in 10 years. Use Table 11-2 to calculate how much she should invest now (in $) at 6% interest, compounded semiannually in order to reach this goal. (Round your answer to...
-
In 1975 the price of a new house was $45,665. In 2020 the price of a new house is $197,222. How much has the price of housing increased on average per year over the time period in percentage terms?...

Study smarter with the SolutionInn App


