Sulfur dioxide is a common preservative in wine; it prevents oxidation and bacterial growth. When SO 2
Question:
Sulfur dioxide is a common preservative in wine; it prevents oxidation and bacterial growth. When SO2 is added to wine, it reacts with water to form an equilibrium system with the bisulfite ion:![]()
In this equilibrium system, SO2 is called “molecular SO2”; in its HSO3– form, it is called “free SO2.” Only molecular SO2 acts as a preservative. The amount of molecular SO2 in the equilibrium system is highly pH dependent—the lower the pH, the more the equilibrium shifts to the left and the greater the amount of free SO2. The recommended amount of free SO2 is 0.8 ppm for white wine and 0.5 ppm for red wine. The table shows the amount of free SO2 required to obtain the correct amount of molecular SO2 as a function of pH for both red and white wine. For dilute solutions such as these, 1 ppm = 1 mg/L.
Study the table and and answer the questions.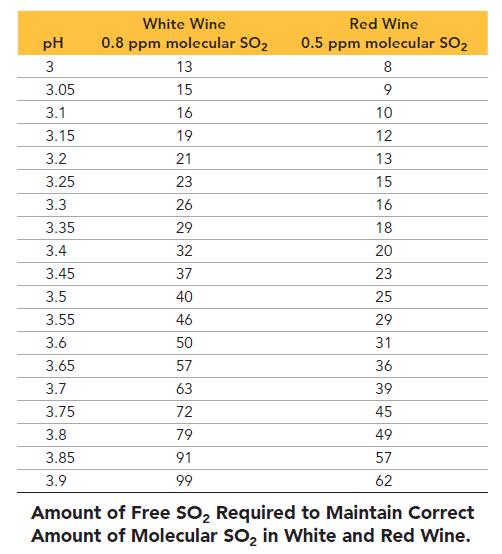
a. A 225-L barrel of white wine has an initial free SO2 concentration of 22 ppm and a pH of 3.70. How much SO2 (in grams) should be added to the barrel to result in the required SO2 level?
b. A 225-L barrel of red wine has an initial free SO2 concentration of 11 ppm and a pH of 3.80. How much SO2 (in grams) should be added to this barrel to result in the required SO2 level?
c. Gaseous SO2 is highly toxic and can be difficult to handle, so winemakers often use potassium metabisulfite (K2S2O5), also known as KMBS, as a source of SO2 in wine. When KMBS is added to wine, the metabisulfite ion (S2O52-) reacts with water to form the bisulfite ion (HSO3–). Write the balanced equation for the reaction that occurs when the metabisulfite ion reacts with water.
d. Determine the percent by mass of SO2 in KMBS.
e. How much KMBS must a winemaker add to the barrels of wine in problems (a) and (b) to achieve the required amount of molecular SO2?
Step by Step Answer:






