The data shown here were collected for the first-order reaction: Use an Arrhenius plot to determine the
Question:
The data shown here were collected for the first-order reaction:![]()
Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction.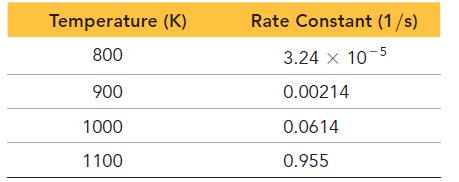
Transcribed Image Text:
N₂O(g) N₂(g) + O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Ea 25...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The tabulated data show the rate constant of a reaction measured at several different temperatures. Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction....
-
The tabulated data show the rate constant of a reaction measured at several different temperatures. Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction....
-
The following data show the rate constant of a reaction measuredat several different temperatures. Temperature (K) Rate Constant (1/s) 300 7.5610 - 2 310 0.221 320 0.605 330 1.56 340 3.79 Part A Use...
-
Can you draw the upper shear zone margin on Figure 16.9? Is it easily definable?
-
Express Delivery is a rapidly growing delivery service. Last year, 80% of its revenue came from the delivery of mailing pouches and small, standardized delivery boxes (which provides a 20%...
-
At the beginning of the current reporting period City Retail Ltd launched a new logo and spent \(\$ 500000\) on new signage for all its premises. The expenditure on signage was originally accounted...
-
What is the mean absolute deviation (MAD)? Why is it useful in forecasting?
-
Given the project network and baseline information below, complete the form to develop a status report for the project at the end of period 4 and the end of period 8. From the data you have collected...
-
Verry urgent solution How can sound be used to measure the temperature of an ocean
-
The rate constant (k) for a reaction was measured as a function of temperature. A plot of ln k versus 1/T (in K) is linear and has a slope of -1.01 * 10 4 K. Calculate the activation energy for the...
-
The rate constant (k) for a reaction was measured as a function of temperature. A plot of ln k versus 1/T (in K) is linear and has a slope of -7445 K. Calculate the activation energy for the reaction.
-
A corporation has 10 shareholders. Nine of the shareholders own 9 percent each of the stock. The tenth shareholder owns the remaining stock. Does the corporation meet the shareholder test as a...
-
Using Houseplan #5 on page 4 of the Measurement supplement(below), determine the cost of pouring the 9 inch thick concreteslab for this home, assuming that the porch will also be on thefoundation....
-
Recall from lecture that Flip-Flap Railway is an old roller coaster that was built in a circle. It has a diameter of 25 ft and riders entered the ride at a speed of 45 mph. At the top of the loop,...
-
Small Fry Design, founded in 1997, is a toy and accessories company that designs and imports products for children. The company's line of merchandise includes teddy bears, musical toys, rattles and...
-
What work trait differences are similar in chart 1 and chart 2? Provide a comment for each of the 4 generations from each chart. Which work trait differences vary from those identified in chart 1 and...
-
Given the ALU design illustrated below, without changing the circuit design, please use the ALU to perform a logic NAND operation. Find out what the control signals should be (i.e. the values of...
-
For the circuit shown in Fig. 11.44, determine the load impedance Z for maximum power transfer (to Z). Calculate the maximum power absorbed by the load. 4 3
-
The rate at which the temperature of an object changes is proportional to the difference between its own temperature and the temperature of the surrounding medium. Express this rate as a function of...
-
Refer to the circuit in Fig. 16.59 . Calculate i(t) for t > 0. 7.5(1 u(t) A i(t) 10 2 10 2
-
Find vo(t) in the circuit of Fig. 16.58 . 1H all 25e u(t) V 2 F=vo(t) 4.5u(t) A vo(t) 4 2 +1
-
Solve for the mesh currents in the circuit of Fig. 16.57 . You may leave your results in the s-domain. 1H 20u(t) V (+ H ( 12
-
Mongo Bongo sells $7,500 of its bongos on credit on a daily basis. Because Mongo Bongo deals with beatniks, it takes 75 days to collect its A/R. (1a) What is the average A/R that is reported on...
-
An equally-weighted portfolio contains eight securities, each with a standard deviation of returns of 50%. If the pairwise correlation of returns for these securities is 0.6, calculate the resulting...
-
Derek borrows $282,135.00 to buy a house. He has a 30-year mortgage with a rate of 4.67%. The monthly mortgage payment is $________. Currency: Round to: 2 decimal places.

Study smarter with the SolutionInn App


