The diagram shows the energy of a reaction as the reaction progresses. Label each blank box in
Question:
The diagram shows the energy of a reaction as the reaction progresses. Label each blank box in the diagram.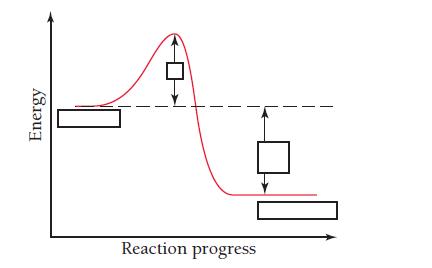
a. Reactants
b. Products
c. Activation energy (Ea)
d. Enthalpy of reaction (ΔHrxn)
Transcribed Image Text:
Energy Reaction progress
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a...View the full answer

Answered By

Amar Kumar Behera
I am an expert in science and technology. I provide dedicated guidance and help in understanding key concepts in various fields such as mechanical engineering, industrial engineering, electronics, computer science, physics and maths. I will help you clarify your doubts and explain ideas and concepts that are otherwise difficult to follow. I also provide proof reading services. I hold a number of degrees in engineering from top 10 universities of the US and Europe.
My experience spans 20 years in academia and industry. I have worked for top blue chip companies.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride...
-
The gas-phase reaction CI(g) + HBr(g) HCI(g) + Br(g) has an overall enthalpy change of - 66 kJ. The activation energy for the reaction is 7 kJ. (a) Sketch the energy profile for the reaction, and...
-
What information must the writer always understand before beginning the submission letter? a. Why the applicant is eligible to apply b. Where the application should be submitted c. How much the...
-
Your independent film production company owns the rights to a script for a rom-com movie. They estimate that the script would cost $30 million to make into a movie, which would have a 35% chance of...
-
Montana Company produces basketballs. It incurred the following costs during the year. Direct materials ........... $14,490 Direct labor .............. $25,530 Fixed manufacturing overhead ........
-
The following table presents the number of years of study in English and language arts and the average SAT writing score for students who took the 2008 SAT exam. a. Compute the least-squares...
-
The following annual charges are incurred in respect of a machine in a shop where manual labour is almost nil and work is done by means of five machines of exactly similar type. (a) Rent and rates...
-
Consider a variation of the PDC decision tree shown in Figure. The company must first decide whether to undertake the market research study. If the market research study is conducted, the outcome...
-
Manufacturing overhead: Select one: O a. includes indirect materials, indirect materials, indirect labor, and factory depreciation. O b. should not be assigned to individual jobs because it bears no...
-
Consider the exothermic reaction: If you were trying to maximize the amount of C 2 H 4 Cl 2 produced, which tactic might you try? Assume that the reaction mixture reaches equilibrium. a. Increasing...
-
Molecular iodine dissociates at 625 K with a first-order rate constant of 0.271 s -1 . What is the half-life of this reaction?
-
Repeat Problem 4 for the case when the temperature of the water tank increases linearly with time at a rate of \(r\). Data From Problem 4: The temperature of a steel sphere with initial temperature...
-
What is the discount rate? PV = 7 0 0 ; t = 5 year period; FV = 1 0 0 0
-
How is planning illustrated in this case story? How is strategic management illustrated in this case story? The new CEO stated that the CEO's job is to give employees a point of view. Explain what...
-
Explain the Following Questions: 1. What essential characteristics exist in a proper understanding of "personal mastery," so that as an individual achieves greater progress in this discipline, they...
-
Few people want to eat discolored french fries. Potatoes are kept refrigerated before being cut for french fries to prevent spoiling and preserve flavor. But immediate processing of cold potatoes...
-
Part 3 of 4 Points: 0.49 of 1 Compute P(X) using the binomial probability formula. Then determine whether the normal distribution can be used to estimate this probability. If so, approximate P(X)...
-
This case should be completed after responding to the requirements in Decision Case 13-2. Refer to the financial statement information of Under Armour and Columbia Sportswear reprinted at the back of...
-
Consider the reaction of acetic acid in water CH 3 CO 2 H(aq) + H 2 O(l) CH3CO 22 (aq) + H 3 O + (aq) where Ka 5 1.8 3 1025. a. Which two bases are competing for the proton? b. Which is the stronger...
-
For the following circuit, specify a minimum set of test vectors for a, b, c, d, and e that will test for all stuck-at faults. Specify the faults tested by each vector. a b
-
Give a minimum set of test vectors that will test for all stuck-at faults in the following circuit. List the faults tested by each test vector. D
-
Find a minimum set of tests that will test all single stuck-at-0 and stuck-at-1 faults in the following circuit. For each test, specify which faults are tested for s-a-0 and for s-a-1. i
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer

Study smarter with the SolutionInn App


