The enthalpy of solution for cesium fluoride is -36.8 kJ/mol. What can you conclude about the relative
Question:
The enthalpy of solution for cesium fluoride is -36.8 kJ/mol. What can you conclude about the relative magnitudes of ΔHsolute and ΔHhydration?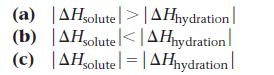
Transcribed Image Text:
(a) AHsolute>|AHhydration (b) AHsolute<| AHhydration (c) |AHsolute = |AHhydration |
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
b You ...View the full answer

Answered By

Rishabh Ojha
During my undergraduate i used to participate as TA (Teaching Assistant) in several electronics and computers subject. I'm passionate about learning Computer Science as my bachelors are in Electronics but i learnt most of the Computer Science subjects on my own which Machine Learning also. At Present, i'm a working professional pursuing my career as a Machine Learning Engineer and i want to help others learn during my free hours, that's all the motivation behind giving tuition. To be frank i have no prior experience of tutoring but i have solved problems on opensource platforms like StackOverflow and github. ~Thanks
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The enthalpy of solution for NaOH is -44.46 kJ/mol. What can you conclude about the relative magnitudes of the absolute values of Hsolute and Hhydration , where Hsolute is the heat associated with...
-
What can you conclude about the relative risk of investing in the United States versus Japan from Figure 7.4?
-
When lithium iodide (LiI) is dissolved in water, the solution becomes hotter. a. Is the dissolution of lithium iodide endothermic or exothermic? b. What can you conclude about the relative magnitudes...
-
1. Resolve Class C 192.168.23.36 /27 2. Design DMZ minimumof 4 servers assigning an IP addressfor all devices 3. List and explain 4 primary servers that will be in the DMZ 4. Resolve Class C 192.168...
-
What is value-chain analysis? What role does it play in strategic cost analysis?
-
(a) How can Fuzzy logic be used to tune a PID controller for effective temperature controll control? (use information from Problem 10.2) (b) What are the Fuzzy input and output, and how do they...
-
A major conclusion in a study reported in Frontiers ofInternational Accounting: An Anthology is that accounting measurements reflected in corporate financial reports represent, in one sense, merely...
-
Bluestem Supply does not segregate sales and sales taxes at the time of sale. The register total for March 16 is $10,388. All sales are subject to a 6% sales tax. Compute sales taxes payable and make...
-
Which of the following would be considered capital expenditures (debit to an asset)? Sales tax on the purchase of equipment Purchase of cleaning supplies to clean the company's microwave Aspecial...
-
You are the revenue manager of a 400-room hotel and you have just received a new group inquiry from your director of sales. Use the Spreadsheet attached "Group Evaluation and Sales Mix Proposal"...
-
A solution is prepared by dissolving 17.2 g of ethylene glycol (C 2 H 6 O 2 ) in 0.500 kg of water. The final volume of the solution is 515 mL. Calculate the concentration of the solution in each...
-
Why do two ideal gases thoroughly mix when combined? What drives the mixing?
-
It is generally recommended that a soft grade wheel be used for grinding hardened steels? Explain why.
-
On January 1, 20X1, Elberta Company issued $50,000 of 4% convertible bonds, in total, into 5,000 shares of Elberta's common stock. No bonds were converted during 20X1. Throughout 20X1 Elberta had...
-
At Vision Club Company, office workers are employed for a 40-hour workweek and are quoted either a monthly or an annual salary (as indicated). Given on the form below are the current annual and...
-
8 Project two 15 UTSA Project two M Question 1 - Project two ChatGPT C chegg.com/homework-he X Course Hero how to take a sxreen shot X +...
-
Charitable purposes: Section 3(1) Charities Act 2011 1. Prevention or relief of poverty 2. Education 3. Religion, now includes: 4. - - A religion which involves belief in more than one god A religion...
-
Jack Price, The finance director of Humpty Doo Investment Ltd ( HDIL ) , is unsure whether he should consolidate some of the investments that the company owns. He has asked your advice as business...
-
Consider a first-to-default basket (FTD) that pays (1 R) upon the default of the first credit in a basket of 3 credits. Protection on the FTD lasts 5 years and payments are made quarterly. The...
-
Find the volume of the described solid S. A frustum of a right circular cone with height h, lower base radius R, and top radius r -r- --R
-
In the fabricated enlargement shown in Fig. 6.29, the pressure at A is 25.6 psig and the pressure at B is 28.2 psig. Calculate the volume flow rate of oil (sg = 0.90). Direction of flow 5-in inside...
-
For the special fabricated reducer shown in Fig. 6.28, the pressure at A is 50.0 psig and the pressure at B is 42.0 psig. Calculate the velocity of flow of water at point B. Flow 1-in inside diameter...
-
For the siphon shown in Fig. 6.27, calculate (a) the volume flow rate of oil from the tank and (b) the pressures at points A, B, C, and D. +B 3,0 m Oil (sg - 0.86) 10.0 m 50-mm OD x 15-mm wall 25-mm...
-
A company purchased $3,400 of merchandise on July 5 with terms 3/10, n/30. On July 7, it returned $600 worth of merchandise. On July 8, it paid the full amount due. The amount of the cash paid on...
-
A corporation that incurs a net operating loss may carry the loss back to earlier years before it can carry the loss forward. True or False
-
Question 2 Prepare the journal entries to record the following transactions on Ivanhoe Company's books using a perpetual inventory system. (If no entry is required, select "No Entry" for the account...

Study smarter with the SolutionInn App


