The propane fuel (C 3 H 8 ) used in gas barbeques burns according to the thermochemical
Question:
The propane fuel (C3H8) used in gas barbeques burns according to the thermochemical equation: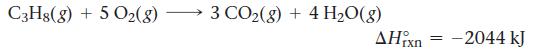
If a pork roast must absorb 1.6 * 103 kJ to fully cook, and if only 10% of the heat produced by the barbeque is actually absorbed by the roast, what mass of CO2 is emitted into the atmosphere during the grilling of the pork roast?
Transcribed Image Text:
C3Hg(g) + 5 O2(g) 3 CO2(g) + 4H2O(g) ΙΧΠ - –2044 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
10...View the full answer

Answered By

Shehar bano
I have collective experience of more than 7 years in education. my area of specialization includes economics, business, marketing and accounting. During my study period I remained engaged with a business school as a visiting faculty member and did a lot of business research. I am also tutoring and mentoring number of international students and professionals online for the last 7 years.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Multinational oil company ExxonMobil faced many challenges related to climate change. Climate change is taking place because of the greenhouse effect. When solar radiation passes through the...
-
In February 19_8, Randy White, president of Arriscraft Corporation (Arriscraft), had just received two requests for a price on two of their marble products. The first request was from a nearby city...
-
Gombas Company decided to analyze certain costs for October of the current year. There was no beginning inventory. Units started into production equaled 14000, units transferred out equal 12000, and...
-
Newell Company has the following internal control procedures over cash disbursements. Identify the internal control principle that is applicable to each procedure. (a) Company checks are prenumbered....
-
Examine the international trends in commercial banking in the past three decades. Analytically account for the trends and, on the basis of your account, comment and make a projection on the future of...
-
What are the single-system and dual-system hypotheses?
-
1. Based on the information provided in the case, do you think the political risk associated with Thailand is higher or lower for a manufacturer of leisure products such as Blades as opposed to, say,...
-
There are various costing methods available for companies to implement. As a company grows, it may become beneficial to consider an alternate costing method. A. Identify an alternative costing method...
-
Consider the following parlor game to be played between two players. Each player begins with three chips: one red, one white, and one blue. Each chip can be used only once. To begin, each player...
-
Charcoal is primarily carbon. Determine the mass of CO 2 produced by burning enough carbon (in the form of charcoal) to produce 5.00 * 10 2 kJ of heat. C(s) + O(8) CO(8) AHxn=-393.5 kJ
-
Titanium reacts with iodine to form titanium(III) iodide, emitting heat. Determine the masses of titanium and iodine that react if 1.55 * 10 3 kJ of heat is emitted by the reaction. 2 Ti(s) + 3 1(g)...
-
Bill and Guilda each own 50 percent of the stock of Radiata Corporation, an S corporation. Guilda's basis in her stock is $25,000. On July 31, 2013, Bill sells his stock, with a basis of $40,000, to...
-
The copper coil placed inside a stove with the purpose of heating water that flows through the coil. The coil is made from copper tube with an OD of 1 2 . 7 0 mm and ID of 1 1 . 0 8 mm . Water enters...
-
Confidence Levels Given specific sample data, such as the data given in Exercise 1, which confidence interval is wider: the 95% confidence interval or the 80% confidence interval? Why is it wider?
-
Yellow M&Ms Express the confidence interval (0.0847, 0.153) in the form of P - E < p < p + E. 12. Blue M&Ms Express the confidence interval 0.255 (+-) 0.046 in the form of P - E < p < p + E.
-
An ideal, noble gas with a mass of 97.2 g at 25 C and a pressure of 608 torr has a volume of 22.7 L. 1. What is the pressure (in atm)? SHOW ALL WORK. 2. What is R (number and units)? 3. What is the...
-
A drug is used to help prevent blood clots in certain patients. In clinical trials, among 4705 patients treated with the drug, 170 developed the adverse reaction of nausea. Construct a 95% confidence...
-
The guidance system design of a satellite places several components in parallel. The system will function as long as at least one of the components is operational. In a particular satellite, 4 such...
-
According to a recent survey, 40% of millennials (those born in the 1980s or 1990s) view themselves more as spenders than savers. The survey also reveals that 75% of millennials view social...
-
Radioactive decay can be thought of as an exercise in probability theory. Imagine that you have a collection of radioactive nuclei at some initial time (N 0 ) and are interested in how many nuclei...
-
First-order decay processes as described in the previous problem can also be applied to a variety of atomic and molecular processes. For example, in aqueous solution the decay of singlet molecular...
-
In a subsequent chapter we will encounter the energy distribution P () = Ae /kT , where P () is the probability of a molecule occupying a given energy state, is the energy of the state, k is a...
-
Group Number: 1. Process Instrumentation Subdivision 1.1. The data Old pressure transmitter equipment production 0 Market values Units Growth Share Initial 100 000 0% 50% Market Units Market Share...
-
Challenge Exercise 7-1 Conklan Company manufactures outdoor fireplaces. For the first 9 months of 2020, the company reported the following operating results while operating at 80% of plant capacity:...
-
Ferris Ltd. is a Canadian controlled private corporation. For the year ending December 31, 2019, its accounting Net Income Before Taxes, as determined under generally accepted accounting principles,...

Study smarter with the SolutionInn App


