The titration of 10.00 mL of HCl solution of unknown concentration requires 12.54 mL of a 0.100
Question:
The titration of 10.00 mL of HCl solution of unknown concentration requires 12.54 mL of a 0.100 M NaOH solution to reach the equivalence point. What is the concentration of the unknown HCl solution in M?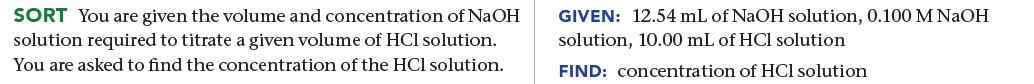
Transcribed Image Text:
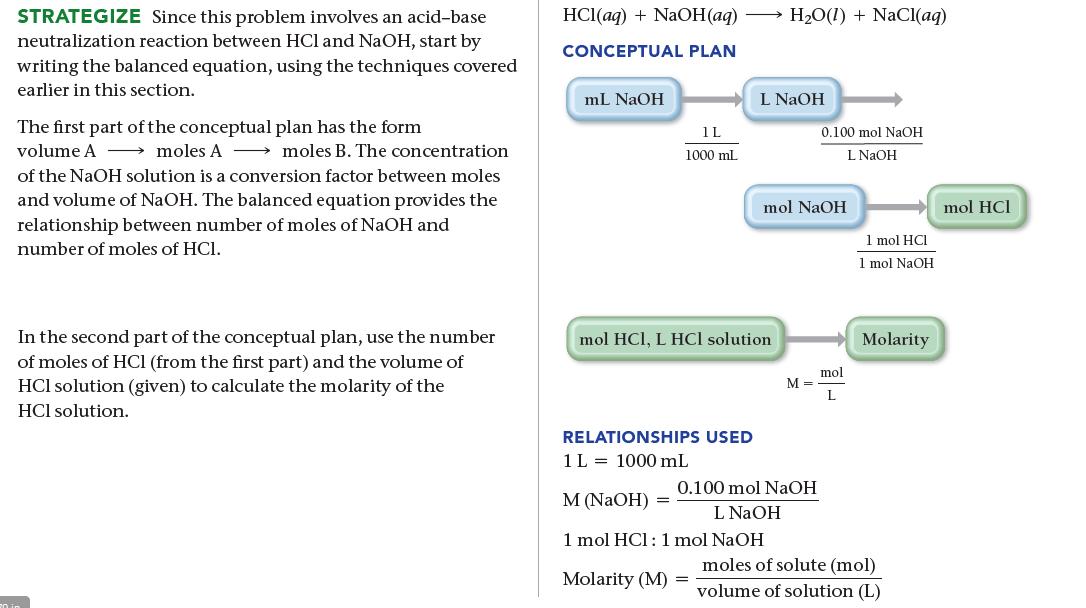
SORT You are given the volume and concentration of NaOH solution required to titrate a given volume of HCl solution. You are asked to find the concentration of the HCl solution. GIVEN: 12.54 mL of NaOH solution, 0.100 M NaOH solution, 10.00 mL of HCl solution FIND: concentration of HCl solution
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
1254 ml NaOH X Molarity 1 L 1000 mL 1 mol HCI 1 mol NaOH 0100 molNaOH LNaOH 125 ...View the full answer

Answered By

Hamza Amjad
Currently I am student in master degree program.from last two year I am tutring in Academy and I tought many O/A level student in home tution.
4.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
The flask contains 25 mL of an unknown diprotic acid aqueous solution that reacts in a 1:2 stochiometric ratio with NaOH. Titrate the solution with NaOH to determine the concentration of the acid....
-
A procedure15 for determining halogens in organic compounds uses an argentometric titration. To 50 mL of anhydrous ether is added a carefully weighed sample (10 - 100 mg) of unknown, plus 2 mL of...
-
dentify a true statement about penetration pricing. It emphasizes a company's current performance but can sacrifice long-term performance. It requires a company to price goods to cover variable costs...
-
Are the inputs and outputs of a production process likely to be different for a home builder than for a cement company? How?
-
True or false? Explain your answer. a. A single-price monopolist sells to some customers that a price-discriminating monopolist refuses to. b. A price-discriminating monopolist creates more...
-
Suppose that the members of your class enter into an agreement under whose terms all would chip in to pay for the damage to any automobile owned by a class member that was damaged in a collision....
-
Cases. Read the following cases. Required: For each case, state whether the action or situation shows a violation of the AICPA Code of Professional Conduct, explain why if it does, and cite the...
-
Secret Reserve will be shown in A ) Profit and Loss Account B ) Profit and Loss Appropriation Account C ) Balance Sheet D ) None of the above
-
The trial balance of Pacilio Security Services Inc. as of January 1, 2020, had the following normal balances: Cash ..................$122,475 Petty cash ................ 100 Accounts receivable...
-
Which statement best describes the difference between the charge of a polyatomic ion and the oxidation states of its constituent atoms? (For example, the charge of NO 3 - is 1, and the oxidation...
-
Write a molecular equation, ionic equation, and net ionic equation for the reaction between aqueous acetic acid (HC 2 H 3 O 2 ) and aqueous potassium hydroxide (KOH).
-
Olson Inc. produces custom-made floor tiles. During June 2001, the following information was obtained relating to operations and production: 1. Direct material purchased on account, $85,000. 2....
-
In the circuit shown below, IE = 120 mA, a = 0.99, a2 = 0.98. Assuming that both transistors are in the active state, answer the following questions: 1. Find IC, IB, IE, IC, and Ic. 2. Find the...
-
Find the open intervals where the function is concave upward or concave downward. Find any inflection points. f(x)=-(x+1)6 Find any critical numbers for f and then use the second derivative test to...
-
Worked Example A two-dimensional incompressible flow is expressed by the velocity functions: u = -2xy y = y = x 1 w=0 Find the pressure field p(x, y) when the pressure at the origin (x=o, y=o) is p.....
-
The following information is available for Blue Spruce Corp. for 2022. Cash used to purchase treasury stock Cash dividends paid $111,592 50,576 Cash paid for interest Net income 51,968 1,077,176...
-
3 4pts Foam k MA C L H W F A box is transported by a truck. Suddenly, the driver uses the brakes and applies 100 N deceleration force to the box. The weight of the box is 20 Kg and its dimensions are...
-
Grain growth is strongly dependent on temperature (i.e., rate of grain growth increases with increasing temperature), yet temperature is not explicitly given as a part of Equation 7.9. (a) Into which...
-
The registrar of a college with a population of N = 4,000 full-time students is asked by the president to conduct a survey to measure satisfaction with the quality of life on campus. The following...
-
Imagine a sound wave with a frequency of 1.10 kHz propagating with a speed of 330 m/s. Determine the phase difference in radians between any two points on the wave separated by 10.0 cm.
-
Given the traveling wave (x, t) = 5.0 exp (-x 2 - bt 2 - 2b xt), determine its direction of propagation. Calculate a few values of and make a sketch of the wave at t = 0, taking = 25 m -2 and b =...
-
Determine which of the following describe traveling waves: (a) (b) (c) (d) Where appropriate, draw the profile and find the speed and direction of motion. (y, t) = ea*y+br2abty) | V(z, t) = A sin (az...
-
Winter Time Adventures is going to annual dividend if $2.61 a share on its common stock next week. This year, the company paid a dividend of $2.50 a share. The company adheres to a constant rate of...
-
Small Factory : -Regular time 8 hours per day. -1 hour daily lunch break. -25 working days per month. -50 workers. -Worker productivity 2.5 units per hour. -sold for $ 150 per unit. -cost of Labor...
-
$500 is invested for 7 years at 10 % p.a. simple interest. How much will the investment be worth after this period

Study smarter with the SolutionInn App


