Two metals, A and B, form a substitutional alloy with the binary phase diagram shown here. Determine
Question:
Two metals, A and B, form a substitutional alloy with the binary phase diagram shown here. Determine the composition and relative amounts of the two phases present at 50% composition and 1500 °C.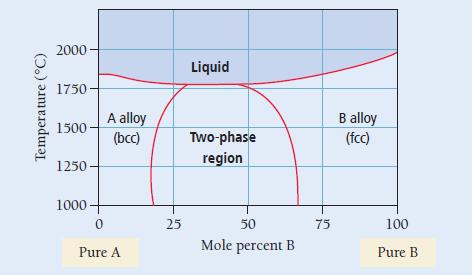
a) The A-rich bcc phase is 80% A and 20% B; the B-rich fcc phase is 37% A and 63% B; the mixture contains more of the A-rich fcc phase.
b) The A-rich bcc phase is 20% A and 80% B; the B-rich fcc phase is 63% A and 37% B; the mixture contains more of the B-rich fcc phase.
c) The A-rich bcc phase is 50% A and 50% B; the B-rich fcc phase is 50% A and 50% B; the mixture contains equal amounts of both phases.
d) The A-rich bcc phase is 80% A and 20% B; the B-rich fcc phase is 37% A and 63% B; the mixture contains more of the B-rich fcc phase.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





