Use covalent Lewis structures to explain why the compound that forms between nitrogen and hydrogen has the
Question:
Use covalent Lewis structures to explain why the compound that forms between nitrogen and hydrogen has the formula NH3. Show why NH2 and NH4 are not stable.
Transcribed Image Text:
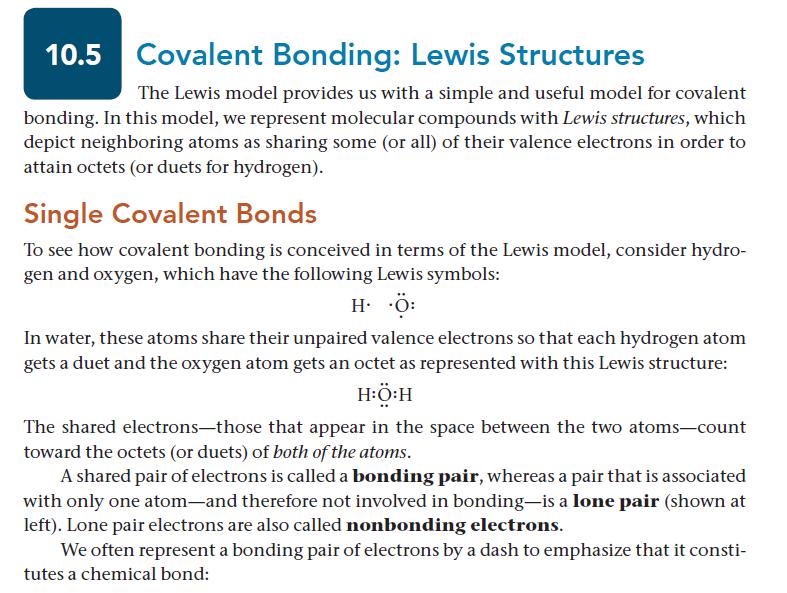
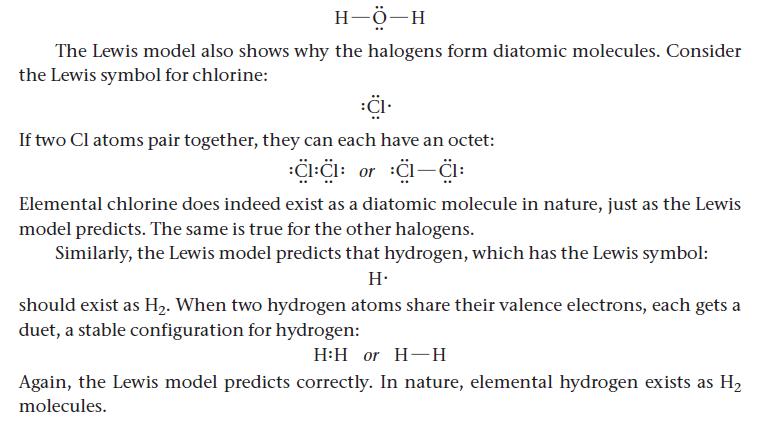
10.5 Covalent Bonding: Lewis Structures The Lewis model provides us with a simple and useful model for covalent bonding. In this model, we represent molecular compounds with Lewis structures, which depict neighboring atoms as sharing some (or all) of their valence electrons in order to attain octets (or duets for hydrogen). Single Covalent Bonds To see how covalent bonding is conceived in terms of the Lewis model, consider hydro- gen and oxygen, which have the following Lewis symbols: HO In water, these atoms share their unpaired valence electrons so that each hydrogen atom gets a duet and the oxygen atom gets an octet as represented with this Lewis structure: HỘ:H The shared electrons-those that appear in the space between the two atoms-count toward the octets (or duets) of both of the atoms. A shared pair of electrons is called a bonding pair, whereas a pair that is associated with only one atom-and therefore not involved in bonding-is a lone pair (shown at left). Lone pair electrons are also called nonbonding electrons. We often represent a bonding pair of electrons by a dash to emphasize that it consti- tutes a chemical bond:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
1 NH3 Nitrogen has five valence electrons and hydrogen has one valence electron Therefore nitrogen c...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write a formula for the compound that forms between potassium and each polyatomic ion. a. Carbonate b. Phosphate c. Hydrogen phosphate d. Acetate
-
Use covalent Lewis structures to explain why each element (or family of elements) occurs as diatomic molecules. a. Hydrogen b. The halogens c. Oxygen d. Nitrogen
-
Use Lewis structures to explain why Br 3 and I 3 are stable, while F 3 is not.
-
2. Two point charges are located at two comers of a triangle as shown. What is the electric potential at the right corner of the triangle? A. 21 10 V C. 4.5 x 10 V B. 3.4 x 10 V D. 6.3 x 10 V 10 em...
-
Describe the meaning of each of the entity-relationship diagrams shown inFigure. Student Number Name Address Student b. Sales account representative has Customens C. Credit card is assigned PIN...
-
Indicate whether each of the following statements is true or false by writing T or F in the answer c olumn. A contract must be completely performed in all its details in order to be considered...
-
If a firm's ROE is low and management wants to improve it, explain how using more debt might help. AppendixLO1
-
An analysis of comparative balance sheets, the current years income statement, and the general ledger accounts of Judd Corp. uncovered the following items. Assume all items involve cash unless there...
-
The outstanding balance after a certain number of payments have been made under the amortization payment plan is equal to the cross out Cross out Select one: a future value of the remaining payments...
-
1. Do you believe Allergans financial information in its press release is useful? Why or why not? 2. Do you believe this kind of information should be subject to audit procedures? If so, what...
-
Write the electron configuration for Ne. Then write the Lewis symbol for Ne and show which electrons from the electron configuration are included in the Lewis symbol.
-
Write the electron configuration for N. Then write the Lewis symbol for N and show which electrons from the electron configuration are included in the Lewis symbol.
-
Data for Mariner Designs, Inc. follow: Requirements 1. Prepare a horizontal analysis of the comparative income statement of Mariner Designs, Inc. Round percentage changes to one decimal place. 2. Why...
-
The accounting equation of Matthew Thomas, attorney, at the beginning of an accounting period is given below, followed by seven transactions whose effects on the accounting equation are shown....
-
Link's Lumber creates pressure-treated utility poles from pine logs bought from several surrounding pine plantations. At the Link's Lumber operation, a batch of 100 pine logs yields 85 utility poles...
-
Draw a mind map with the word leadership in the centre. You could start the map here and build or add to it as you progress through the book or over the trajectory of your studies.
-
The performance of a project was evaluated 10 weeks after its start. Table 15 gives the relevant information. a. On the same Gantt chart, show the project plan and the project progress, and discuss...
-
The following balances were reported in the financial statements for Nadir Company. Required 1. Compute the following ratios for 2019 and 2018 for Nadir Company. a. Return on sales ratio b. Current...
-
A cylindrical specimen of some alloy 8 mm (0.31 in.) in diameter is stressed elastically in tension. A force of 15,700 N (3530 lbf) produces a reduction in specimen diameter of 5 10-3 mm (2 10-4...
-
The age-old saying for investing is "buy low and sell high," but this is easier said than done. Investors who panic about falling prices sell their investments, which in turn lowers the price and...
-
Refer to Fig. 4.41. 6 in 18 in 30 in Triangular window Water 50 20 in
-
Figure 4.42 shows a gasoline tank filled into the filler pipe. The gasoline has a specific gravity of 0.67. Calculate the total force on each flat end of the tank and determine the location of the...
-
If the tank in Fig. 4.42 is filled just to the bottom of the filler pipe with gasoline (sg = 0.67), calculate the magnitude and location of the resultant force on the flat end. 375 mm 300 mm-...
-
Q) A stock price is currently $90. Over each of the next two 6-month periods, it is expected to go up by 10% or down by 10%. The risk-free interest rate is 8% per annum with continuous compounding....
-
A couple who borrow $80,000 for 30 years at 7.2%, compounded monthly, must make monthly payments of $543.03. (Round your answers to the nearest cent.) (a) Find their unpaid balance after 1 year. $...
-
You have been hired by Internal Business Machines Corporation (IBM) in their capital budgeting division. Your first assignment is to determine the free cash flows and NPV of a proposed new type of...

Study smarter with the SolutionInn App


