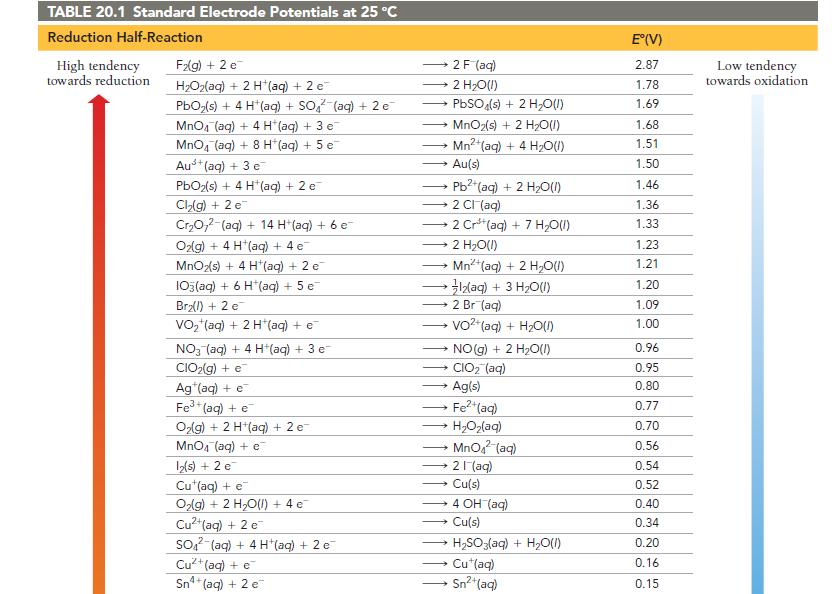
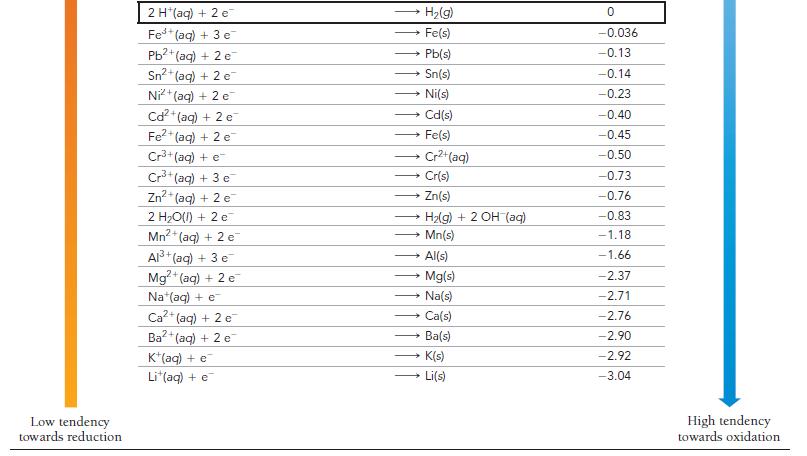
Use Table 20.1 to calculate G for the reaction. 2 MnO4 (aq) + Cd(s) - a) +30.9
Question:
Use Table 20.1 to calculate ΔG° for the reaction.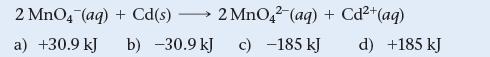
Transcribed Image Text:
2 MnO4 (aq) + Cd(s) - a) +30.9 kJ 2 MnO42 (aq) + Cd²+ b) -30.9 kJ c) -185 kJ (aq) d) +185 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 20% (5 reviews)
c...View the full answer

Answered By

Amar Kumar Behera
I am an expert in science and technology. I provide dedicated guidance and help in understanding key concepts in various fields such as mechanical engineering, industrial engineering, electronics, computer science, physics and maths. I will help you clarify your doubts and explain ideas and concepts that are otherwise difficult to follow. I also provide proof reading services. I hold a number of degrees in engineering from top 10 universities of the US and Europe.
My experience spans 20 years in academia and industry. I have worked for top blue chip companies.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Carbon disulfide (CS2) is a toxic, highly flammable substance. The following thermodynamic data are available for CS2(l) and CS2(g) at 298 K: (a) Draw the Lewis structure of the molecule. What do you...
-
GSSG + NADPH + H+ 2GSH + NADP+ (a) Calculate G for the glutathione reductase reaction in the direction shown, using E values from Table 14.1. (b) Suppose that a cell contained an isoform of...
-
Using data from Appendix C, calculate G for the following reactions. Indicate whether each reaction is spontaneous at 298 K under standard conditions. 2 SO2(g) + O2(g) 2 SO3(g) NO2(g) + N20(g)-3N0(g)...
-
Solve the inequality. Write the solution in interval notation. |15 = x < 7
-
Books-For-All is a well-established chain of 20 bookstores in western Ohio. In recent years, the company has grown rapidly, adding five new stores in regional malls. The manger of each store selects...
-
Continuing with the ECCO company from Problem 4-76, when a customer returns one of the alarms under warranty, the quality manager logs data on the product. From the data available in the Ecco file,...
-
What benefits do customers receive in return for the sacrifice they make when buying a membership at Planet Fitness? Planet Fitness: Pricing for Success22 How does going completely against the grain...
-
Consider the following account balances (in thousands) for the Rouse Company: Required: 1. Prepare a schedule for the cost of goods manufactured for 2013. 2. Revenues for 2013 were $ 265 million....
-
Bonds are issued on June 1, 2021 that have interest payment dates of April 1 and October 1. Bond interest expense for the year ended December 31, 2021, is for a period of three months four months...
-
Consider the reaction at 298 K: Calculate G rxn under these conditions: Is the reaction more or less spontaneous under these conditions than under standard conditions? 2 NO(g) + O(8) O(g) 2 NO(8)...
-
Arrange these gases in order of increasing standard molar entropy: SO 3 , Kr, Cl 2 . (a) Kr < Cl < SO3 (c) SO3 Cl < Kr (b) Kr < SO3 < Cl (d) Cl < Kr < SO3
-
During the month of June, Danielle's Boutique had cash sales of R$265,000 and credit sales of R$153,700, both of which include the 6% sales tax that must be remitted to the government by July 15....
-
Question (4) seen, 20 vehicles/km moving at 100 km/h and 30 vehicles/km traveling at 120 km/h. Two successive videos showing stationary traffic on the road were examined. Two groups of platoons were...
-
?In civil engineering, what is the main use of a slump test in concrete technology?
-
Explain the process of compression resin transfer molding(CRTM)?in composite manufacturing. What are the benefits of using CRTM for producing composite structures?
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Discuss the principles of geotechnical engineering in slope stability analysis. How can engineers assess slope stability, mitigate landslide risks, and design effective stabilization measures to...
-
Answer these questions about consolidation accounting: 1. Define "parent company." Define "subsidiary company." 2. How do consolidated financial statements differ from the financial statements of a...
-
we have to compute the letter grades for a course. The data is a collection of student records stored in a file. Each record consists of a name(up to 20 characters), ID (8 characters), the scores of...
-
Coaxial cables are widely used with audio-visual technology, electronic instrumentation, and radio broadcasting, because they minimize interference with or from signals traveling on the cable....
-
A thin rod of length L carries charge Q distributed uniformly over its length. (a) Show that the potential in the plane that perpendicularly bisects the rod is given by where r is the perpendicular...
-
Two closely spaced square conducting plates measure 10 cm on a side. The electric-field energy density between them is 4.5 kJ/m 3 . Whats the charge on the plates?
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


