Use the mass spectrum of rubidium to determine the atomic mass of rubidium. Intensity % 100% 50%-
Question:
Use the mass spectrum of rubidium to determine the atomic mass of rubidium.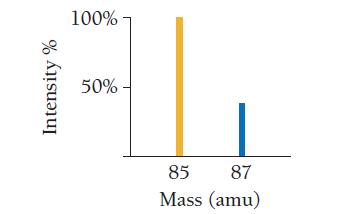
Transcribed Image Text:
Intensity % 100% 50%- 85 87 Mass (amu)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
The image you sent shows the mass spectrum of rubidium The mass spectrum shows two pea...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Mass spectrometry is one of the most versatile and powerful tools in chemical analysis because of its capacity to discriminate between atoms of different masses. When a sample containing a mixture of...
-
Use the mass spectrum of europium to determine the atomic mass of europium. Intensity % 100% 50% - 151 153 Mass (amu)
-
Mass spectrometry is more often applied to molecules than to atoms. We will see in Chapter 3 that the molecular weight of a molecule is the sum of the atomic weights of the atoms in the molecule. The...
-
The e-commerce business in China has entered a golden period, with transaction volume of online trading reaching 21.86 billion yuan (US$2.64 billion) in 2004. With 94 million Internet users, more...
-
Gollum Co. has no debt. Its cost of capital is 9.5 percent. Suppose Gollum coverts to a debt-equity ratio of 1.0. The interest rate on the debt is 7.2 percent. Ignoring taxes, what Gollums new cost...
-
A four-wheel antilock automobile braking system uses electronic feedback to control automatically the brake force on each wheel [15]. A block diagram model of a brake control system is shown in...
-
Why might a multinational corporation decide to borrow in a country such as Brazil, where interest rates are high, rather than in a country like Switzerland, where interest rates are low? AppendixLO1
-
Presented below are a number of independent situations. Instructions For each individual situation, determine the amount that should be reported as cash. If the item(s) is not reported as cash,...
-
You have risen through the ranks of a coffee comany, from the lowly green-apron barista to the coveted black apron, and all the way to CFO. A quick internet check shows that your company's beta is...
-
Arndt, Inc., reported the following for 2024 and 2025 ($ in millions): a. Expenses each year include $30 million from a two-year casualty insurance policy purchased in 2024 for $60 million. The cost...
-
How many sulfur atoms are there in 5.52 mol of sulfur?
-
Silicon has three naturally occurring isotopes (Si-28, Si-29, and Si-30). The mass and natural abundance of Si-28 are 27.9769 amu and 92.2%, respectively. The mass and natural abundance of Si-29 are...
-
Discuss the sources of heat wastage and its potential applications.
-
The test statistic of z = - 2.93 is obtained when testing the claim that p < 2/ 3. This is a left-tailed test. Using a 0.01 significance level, complete parts (a) and (b). a. Find the critical...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 C. Determine the magnitude and direction of the electric field along the axis of the rod at a point 32.0 cm from its center....
-
Hello need help with this problem. The transactions relating to the formation of Blue Company Stores Incorporated, and its first month of operations follow. a. The firm was organized and the...
-
At the beginning of the year, the net assets of Shannon Company were $492,600. The only transactions affecting stockholders equity during the year were net income of $70,200 and dividends of $15,400....
-
If you assume the average length of linker DNA is 50 bp, approximately how many nucleosomes are found in the haploid human genome, which contains 3 billion bp?
-
The manager of a local convenience store is expanding his line of small toy items. To price these new items, the manager is looking at the prices being charged by competing retailers in his area. For...
-
Predict the major product of the reaction between 1-butanol and: (a) PBr 3 (b) SOCl 2 , py (c) HCl, ZnCl 2 (d) H 2 SO 4 , heat (e) PCC, CH 2 Cl 2 (f ) Na 2 Cr 2 O 7 , H 2 SO 4 , H 2 O (g) Li (h) NaH...
-
What can you conclude about the ratio of fugacity to pressure for N 2 ,H 2 , and NH 3 at 500 bar using the data in Figure 7.10? Figure 7.10 1.5 H2, 1.4 N2 1.3 1.2 1.1 1.0 100 200 300 400 500 600 700...
-
A system consisting of 82.5 g of liquid water at 300. K is heated using an immersion heater at a constant pressure of 1.00 bar. If a current of 1.75 A passes through the 25.0 ohm resistor for 100. s,...
-
Sierra Company manufactures soccer balls in two sequential processesCutting and Sutching. All direct materials enter production at the beginning of the cutting process. The following information is...
-
Whenever alimony and child support payments are both required as the result of a divorce decree and the full payment is not made, child support is always considered to have been paid first. Choose...
-
MAJOR CASE STUDY You have commenced work at Alfreds Accountants, and Alfred has given you a series of tasks to perform. The first task is as follows: Alfred hands you a pre-adjustment trial balance...

Study smarter with the SolutionInn App


