When 1.010 g of sucrose (C 12 H 22 O 11 ) undergoes combustion in a bomb
Question:
When 1.010 g of sucrose (C12H22O11) undergoes combustion in a bomb calorimeter, the temperature rises from 24.92 °C to 28.33 °C. Find ΔErxn for the combustion of sucrose in kJ/mol sucrose. The heat capacity of the bomb calorimeter, determined in a separate experiment, is 4.90 kJ/°C.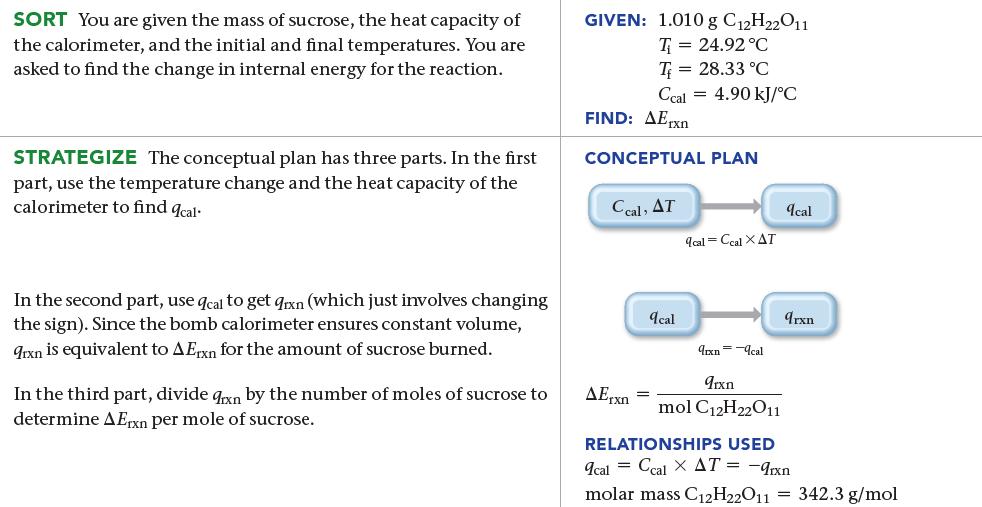
Transcribed Image Text:
SORT You are given the mass of sucrose, the heat capacity of the calorimeter, and the initial and final temperatures. You are asked to find the change in internal energy for the reaction. STRATEGIZE The conceptual plan has three parts. In the first part, use the temperature change and the heat capacity of the calorimeter to find 9cal. In the second part, use qcal to get aixn (which just involves changing the sign). Since the bomb calorimeter ensures constant volume, 9rxn is equivalent to A Exn for the amount of sucrose burned. In the third part, divide qxn by the number of moles of sucrose to determine AErxn per mole of sucrose. GIVEN: 1.010 g C12H22011 T= 24.92 °C T= 28.33 °C Ccal FIND: AErxn CONCEPTUAL PLAN Ccal, AT AErxn = 4.90 kJ/°C qcal qcal Ccal XAT 9rxn=cal 9rxn mol C12H22011 qcal 9rxn RELATIONSHIPS USED 9cal = ccal X AT = -9rxn molar mass C₁2H22011 = 342.3 g/mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
T T T 2833 C 2492 C 341 C qcal Ccal X AT ...View the full answer

Answered By

Arshad Ahmad
Well, I am really new to tutoring but I truly believe a good student can be a better teacher. I have always been a topper at school. I passed my Chartered Accountancy at a very young age of 23, a rare feat for most of the students. I am really dedicated to whatever work I do and I am very strict regarding deadlines. i am always committed and dedicated to whatever work allotted to me and I make sure it is completed well within deadline and also I try to give my best in whatever I do. Hope we will have a good time studying together.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Define pay iniquity
-
When 1.10 g of ethanol (C 2 H 6 O) undergoes combustion in a bomb calorimeter, the temperature rises from 22.32C to 29.48C. Find E rxn for the combustion of ethanol in kJ/mol. The heat capacity of...
-
When 0.514 g of biphenyl (C 12 H 10 ) undergoes combustion in a bomb calorimeter, the temperature rises from 25.8 C to 29.4 C. Find E rxn for the combustion of biphenyl in kJ/mol biphenyl. The heat...
-
Consider a social network where people are represented as vertices and friendships as edges. If there are 1 0 people in the network and each person is friends with 3 others, calculate the total...
-
Morey Company has just completed its first year of operations. The companys absorption costing income statement for the year appears below: The companys selling and administrative expenses consist of...
-
Figure P11-70 shows an interconnection of three basic OP AMP modules. (a) Does this interconnection involve loading? (b) Find the overall transfer function of the interconnection and locate its poles...
-
What are the characteristics of companies likely to use a job-order costing system?
-
Danya company has created a new software application for PCs. Its costs during research and development were $250,000. Its costs after the working program was developed were $175,000. Although the...
-
Mike is the trustee of an investment trust. He receives $100,000 from Investor 1 and $100,000 from Investor 2 (in that order), which he holds in a single bank account. In breach of trust, Mike then...
-
While James Craig and his former classmate Paul Dolittle both studied accounting at school, they ended up pursuing careers in professional cake decorating. Their company, Good to Eat (GTE),...
-
From a molecular viewpoint, where does the energy absorbed in an endothermic chemical reaction go? Why does the reaction mixture undergo a decrease in temperature even though energy is absorbed?
-
A 32.5 g cube of aluminum initially at 45.8 C is submerged into 105.3 g of water at 15.4 C. What is the final temperature of both substances at thermal equilibrium? (Assume that the aluminum and the...
-
Local 182, International Union of Electrical Workers, went on strike at the General Electric (GE) plant in Hickory, North Carolina, on October 24. The union immediately established a picket line in...
-
f. The coordinates of two points A and B are (1, 2) and (5,7) respectively. Find the equation and slope of the line AB. g. Find the rate of change of the area of a circle w.r.t its radius r when r =...
-
1. Sketch the anticipated pattern of cracks on the beam structure shown below. Assume that the structure is adequately reinforced for the load shown, and that the loads are large enough to cause...
-
Estimate the hydrogen consumption required to completely remove the sulfur from a hydrotreater feedstock and to reduce the nitrogen content of the product to 15 ppm by weight. The 48.5 API naphtha...
-
2. Consider the following kinds of information, and suggest the most appropriate data type to store or represent each: Information Suggested Data Type String A person's name A person's age in years A...
-
Steam at 32 MPa, 520C enters the first stage of a supercritical reheat cycle including three turbine stages. Steam exiting the first-stage turbine at pressure p is reheated at constant pressure to...
-
The vulcanization of polyisoprene is accomplished with sulfur atoms according to Equation 15.4. If 57 wt% sulfur is combined with polyisoprene, how many cross links will be associated with each...
-
A business had revenues of $280,000 and operating expenses of $315,000. Did the business (a) Incur a net loss (b) Realize net income?
-
Consider the reaction: 2 COF 2 (g) CO 2 (g) + CF 4 (g) Kc = 2.00 In an equilibrium mixture, the concentration of COF 2 is 0.35 M and the concentration of CO 2 is 0.144 M. What is the equilibrium...
-
Consider the reaction: N 2 O 4 (g) 2 NO 2 (g) Kc = 0.36 A reaction mixture initially contains [N 2 O 4 ] = 0.100 M. Find the equilibrium concentrations of N 2 O 4 and NO 2 .
-
Consider the reaction: 2 H 2 S (g) 2 H 2 (g) + S 2 (g) Kc = 1.67 10 -7 A reaction mixture initially contains [H 2 S] = 0.010 M. Find the equilibrium concentrations of H 2 and S 2 .
-
On October 1, 2020 Macklin Corporation issued 5%, 10-year bonds with a face value of $6,000,000 at 104. Interest is paid on October 1 and April 1, with any premiums or discounts amortized on a...
-
You have been hired by Internal Business Machines Corporation (IBM) in their capital budgeting division. Your first assignment is to determine the free cash flows and NPV of a proposed new type of...
-
Suppose that the dollar-mark 6 months forward rate is $1.275/Mark. Suppose that the dollar-mark forward premium is 5%. Calculate the spot rate, $1=Mark_______ work to 4 decimal places.

Study smarter with the SolutionInn App


