Question:
When a suspected drunk driver blows 188 mL of his breath through the fuel-cell breathalyzer described in Section 20.7, the breathalyzer produces an average of 324 mA of current for 10 s.
Assuming a pressure of 1.0 atm and a temperature of 25 °C, what percent (by volume) of the driver’s breath is ethanol?
Transcribed Image Text:
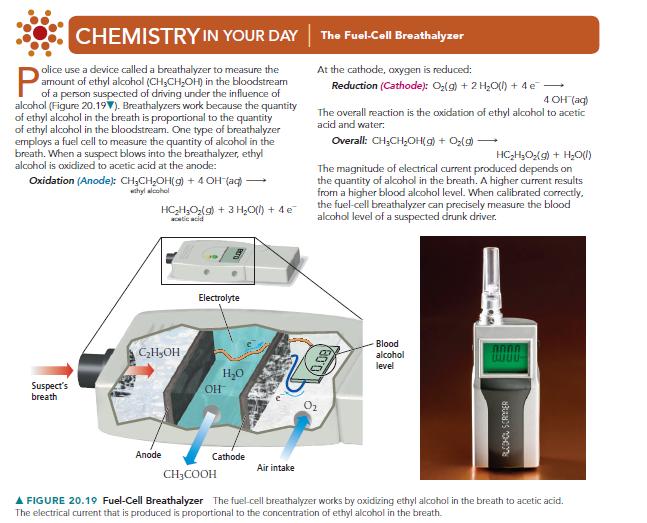
CHEMISTRY IN YOUR DAY The Fuel-Cell Breathalyzer olice use a device called a breathalyzer to measure the amount of ethyl alcohol (CH3CHOH) in the bloodstream alcohol (Figure 20.197). Breathalyzers work because the quantity of ethyl alcohol in the breath is proportional to the quantity of ethyl alcohol in the bloodstream. One type of breathalyzer employs a fuel cell to measure the quantity of alcohol in the breath. When a suspect blows into the breathalyzer, ethyl alcohol is oxidized to acetic acid at the anode: Oxidation (Anode): CHCHOH(g) + 4 OH(aq) whyl alcohol HC2H3O2(g) + 3 HO(l) + 4 e acetic acid At the cathode, oxygen is reduced: Reduction (Cathode): O2(g) + 2 H2O(l) + 4 e 4 OH(aq) The overall reaction is the oxidation of ethyl alcohol to acetic acid and water: Overall: CHCHOH(g) + O2(g) + HC2H3O2(g) + H2O(l) The magnitude of electrical current produced depends on the quantity of alcohol in the breath. A higher current results from a higher blood alcohol level. When calibrated correctly. the fuel-cell breathalyzer can precisely measure the blood alcohol level of a suspected drunk driver. Suspect's breath CHOH Electrolyte HO Anode Cathode Air intake CH3COOH 800 Blood alcohol level ALCOHOL SCRIER FIGURE 20.19 Fuel-Cell Breathalyzer The fuel-cell breathalyzer works by oxidizing ethyl alcohol in the breath to acetic acid. The electrical current that is produced is proportional to the concentration of ethyl alcohol in the breath.








