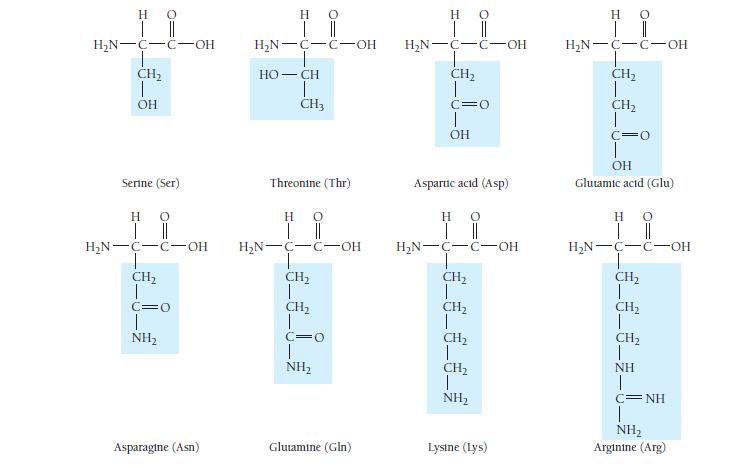
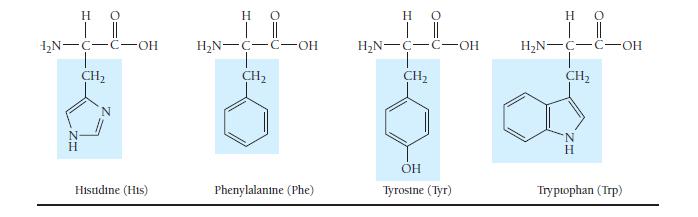
Which amino acids in Table 23.3 are most likely to be involved in hydrophobic interactions? TABLE 23.3
Question:
Which amino acids in Table 23.3 are most likely to be involved in hydrophobic interactions?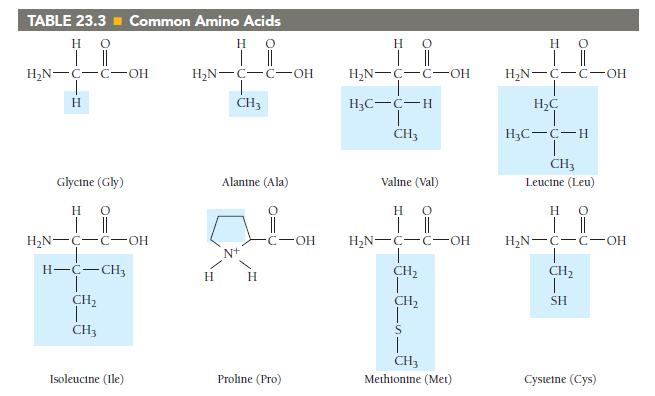
Transcribed Image Text:
TABLE 23.3 Common Amino Acids Η Ο || | Η Ο || | H₂N-C-C-OH H Glycine (Gly) HO | || H₂N-C-C-OH H-C-CH3 CH₂ CH3 Isoleucine (Ile) H₂N-C-C-OH T CH3 H Alanine (Ala) H C-OH Proline (Pro) Η | H₂N-C-C-OH H₂C-C-H T CH3 Ο || Valine (Val) HO | || H₂N-C-C-OH !_ ______ € CH₂ CH₂ CH3 Methionine (Met) H 0 H₂N-C-C-OH H₂C H₂C-C-H CH3 Leucine (Leu) H 0 | || H₂N-C-C-OH CH₂ T SH Cysteine (Cys)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Valine ...View the full answer

Answered By

ALBANUS MUTUKU
If you are looking for exceptional academic and non-academic work feel free to consider my expertise and you will not regret. I have enough experience working in the freelancing industry hence the unmistakable quality service delivery
4.70+
178+ Reviews
335+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which amino acids in Table 17.1 have nonpolar R groups? Highly polar groups? Relatively flat R groups? Table 17.1 QUESTION CONTINUE TO NEXT PAGE Table 17.1 Names and Formulas of the Common Amino...
-
Which of the amino acids in Table 27.1 have more than one stereogenic center? Table 27.1 TABLE 27.1 -Amino Acids Found in Proteins (Continued) Abbreviation Structural formula* Name Amino acids with...
-
The debt of INKA consists of two bonds. The first bond was issued in 2011, with a 15-year maturity and a 6% coupon rate. The second bond was issued in 2016, with a 10-year maturity and a 8% coupon...
-
i. Find a. b. ii. Use the trapezium rule with 2 intervals to estimate the value of giving your answer correct to 2 decimal places. +6 e2x + 6 e2 dx,
-
In chronological order, the inventory, purchases, and sales of a single product for a recent month are as follows: Using the periodic inventory system, compute the cost of ending inventory , cost of...
-
Women diagnosed with breast cancer whose tumors have not spread may be faced with a decision between two surgical treatments mastectomy (removal of the breast) or lumpectomy ( only the tumor is...
-
The median freshman class size at a California college is less than or equal to 400.
-
A metal sign for a car dealership is a thin, uniform right triangle with base length b and height h. The sign has mass M. (a) What is the moment of inertia of the sign for rotation about the side of...
-
A. a decrease in days" sales in receivables from year to year B. Aaysi" suales in receivables grow fastor than for competaors C. an increase in cash flows D. a highh itmentory fumover
-
The amino acid alanine has the condensed structural formula shown here: Determine the VSEPR geometry about each internal atom and make a three-dimensional sketch of the molecule. NH,CH(CH3)COOH
-
What is the difference between a codon and a nucleotide? A codon and a gene?
-
Heat engine X takes in four times more energy by heat from the hot reservoir than heat engine Y. Engine X delivers two times more work, and it rejects seven times more energy by heat to the cold...
-
From Hoffman, what are the symptoms of autism and ADHD? From the Mayo Clinic, what are the causes and risk factors for autism and ADHD? What are treatment options for these disorders? Hofmann, S. G....
-
Brooks, a participant in the Zappa retirement plan, has requested a second plan loan. His vested account balance is $80,000. Brooks borrowed $27,000 eight months ago and still owes $18,000 on that...
-
Suppose that Angelina and Brad own the only two professional photography stores in town. Each must choose between a low price and a high price for senior photo packages. The annual economic profit...
-
based on the article How Chili's Is Prepping for Tough Times, Starting With the Fries by Heather Haddon. What is corporate social responsibility, and what is one way that Chili's can better pursue...
-
QUESTION 2 (20 marks) CLO 5 a. Explain what the following ratios indicate to a firm: (i) Acid Test Ratio (ii) Return on Capital Employed (ROCE) (iii) Debtors Collection Period (iv) Working Capital (2...
-
At December 31, 2016, Pioneer Corporation reported the stockholders' equity accounts shown here (with dollar amounts in millions, except per-share amounts). Common stock $3.00 par value per share, 22...
-
1. Following are information about Alhadaf Co. Cost incurred Inventory Purchases Sales Adverting expense Salary Expense Depreciation Beginning Inventory Ending Inventory Amount 118,000 350.000 90,000...
-
Light from the star Vega has an intensity of about 2 x 10 8 W/m 2 . If Vega emits radiation with the same power as our Sun, how far is Vega from the Earth?
-
Unpolarized light with an electric field amplitude of 0.25 V/m is incident on a polarizer. What is the electric field amplitude of the transmitted light?
-
Linearly polarized light propagating along the y-direction is incident on a polarizer whose axis is parallel to the z-direction. If the intensity of the transmitted light is equal to 35% of the...
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


