Without doing any calculations, determine the signs of S sys and S sur r for each chemical
Question:
Without doing any calculations, determine the signs of ΔSsys and ΔSsurr for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high temperatures), if any, the reaction is spontaneous.
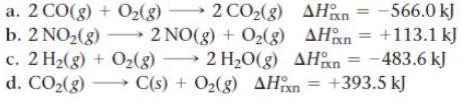
Transcribed Image Text:
a. 2 CO(g) + O(g) 2 CO(g) b. 2 NO(g) 2NO(g) + O(g) AH AH = -566.0 kJ = +113.1 kJ c. 2 H(g) + O(g) 2HO(g) AHxn=-483.6 kJ d. CO(g) C(s) + O(g) AHxn = +393.5 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Without doing any calculations we can determine the signs of Ssys and Ssurr for each chemical reacti...View the full answer

Answered By

Evans Cherono
I am an Information Technology Graduate and willing to work on any computer science or IT work to ensure I do my best all the time.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Without doing any calculations, determine the signs of S sys and S sur r for each chemical reaction. In addition, predict under what temperatures (all temperatures, low temperatures, or high...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
3. Questions A venture capitalist (VC) is willing to invest 100m for 20% ownership of a start-up that is looking to achieve scale. All existing shares are common shares, and this deal would result in...
-
Sampling presents some major problems in market research. Discuss.
-
On January 1, Applied Technologies Corporation (ATC) issued $ 500,000 in bonds that mature in 10 years. The bonds have a stated interest rate of 10 percent. When the bonds were issued, the market...
-
Income tax is provided only in cost accounts.
-
Granite Stone Creamery sold ice cream equipment for $ 16,000. Granite Stone originally purchased the equipment for $ 90,000, and depreciation through the date of sale totaled $ 71,000. What was the...
-
Question 3 Not yet answered Onandomba Pty (Ltd) provides skilled labour to the construction of low-cost apartments. They have recently been asked by a builder NHE to bid for a kitchen fitting...
-
Without doing any calculations, determine the sign of S sys for each chemical reaction. a. 2 KClO 3 (s) 2 KCl(s) + 3 O 2 ( g) b. CH 2 = CH 2 ( g) + H 2 ( g) CH 3 CH 3 (g) c. Na(s) + 1/2 Cl 2 (g) ...
-
Without doing any calculations, determine the sign of S sys for each chemical reaction. a. Mg(s) + Cl(g) MgCl(s) b. 2 HS(g) + 3 O(g) 2 HO(g) + 2 SO(g) c. 203(g) d. HCI(g) + NH3(g) NH4Cl(s) 3 O(g)
-
An air table is used to study the elastic motion of flexible spacecraft models. Pressurized air escaping from numerous small holes in the horizontal surface provides a supporting air cushion which...
-
14. (3 points) Using our model of the egg and vinegar (part one), what is the causal account in this model? Refer to your model and work through the components and relationships that cause this...
-
D. Rewrite the following statements from an "I Approach" to a "You Approach". (5 marks) 1. We have not received your signed invoice so we cannot process payment. 2. I need to know what type of model...
-
AP Precalculus Unit 1 Study Guide: Polynomials Name: Date: 19. The graph of an ODD function is given for the interval -6x0. Use the properties of odd functions to sketch the graph of the function on...
-
Step 1 of 5: Determine the lower class boundary for the second class. Scores on a Test Class Frequency 30-47 11 48-65 14 66-83 11 84-101 9 102-119 12
-
Write a 1,500-2,000-word evaluation paper using the following instructions to complete this assignment. Go to the FBI Uniform Crime Reporting Program website (See link in the Class Resources). Search...
-
Morgan Stanley is a leading investment bank founded in 1935. The company's fiscal year ends December 31, 2013, and it filed its financial statements with the SEC on February 25, 2014. On February 5,...
-
Government is advised to tax goods whose demand curves are inelastic if the goal is to raise tax revenues. If the goal is to discourage consumption, then it ought to tax goods whose demand curves are...
-
A long narrow slit 0.10 mm wide is illuminated by light of wavelength 500 nm coming from a point source 0.90 m away. Determine the irradiance at a point 2.0 m beyond the screen when the slit is...
-
A long horizontal narrow slit of width 0.70 mm is illuminated with 600-nm light. A point-P, 1.0 m away from the aperture screen, is opposite the lower edge of the screen. If 100 W/m 2 arrives at P...
-
A long narrow horizontal opaque rectangular object of width 0.70 mm is illuminated by 600-nm light. Consider a point-P, at the level of the lower edge of the object, 1.0 m from it. Determine the...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...

Study smarter with the SolutionInn App


