Calculate the molar mass of the following substances. a. 0 OP b. Ca3(PO4)2 c. NaHPO4
Question:
Calculate the molar mass of the following substances.
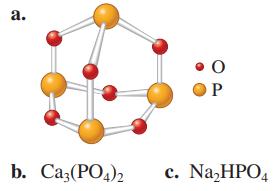
Transcribed Image Text:
a. 0 OP b. Ca3(PO4)2 c. Na₂HPO4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
For molar mass calculation we should add the sum of atomic masses of a...View the full answer

Answered By

Hillary Waliaulah
As a tutor, I am that experienced with over 5 years. With this, I am capable of handling a variety of subjects.
5.00+
17+ Reviews
30+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Calculate the molar mass of the following substances: (a) Li2 CO3, (b) CS2, (c) CHCl3 (chloroform), (d) C6H8O6 (ascorbic acid, or vitamin C), (e) KNO3, (f) Mg3N2.
-
Elaborate figures given below Price per pair (dollars) 105 90 75 60 45 30 15 0 10 20 30 I E Equilibrium IS 40 50 60 70 Quantity of tennis shoes (thousands of pairs per year) 80 90 100 D
-
Richards and Willard determined the molar mass of lithium collected the following data. 6 (a) Find the mean molar mass determined by these workers (b) Find the median molar mass (c) Assuming that the...
-
To enable a process to Wait(condition) within the monitor, which one of the following is TRUE? All of mentioned Semaphore must be used Condition variables must be used as boolean objects A condition...
-
On January 1, 2008, a company agrees to pay $20,000 in three years. If the annual interest rate is 10%, determine how much cash the company can borrow with this agreement.
-
Loblaw Manufacturing has asked you to create a cash budget in order to determine its borrowing needs for the June to October period. You have gathered the following information. April and May sales...
-
LO5 Erin, a single taxpayer, has a taxable income of $103,000 in the current year before considering the following capital gains and losses: In addition, Erin has an $8,000 long-term capital loss...
-
Neveranerror Inc. was organized on June 2, 2010, by a group of accountants to provide accounting and tax services to small businesses. The following transactions occurred during the first month of...
-
Blue & Gold Pty Ltd borrows $222 000 cash from Bankwest Ltd in 1986 and intends to pay it back in 2021. How would the transaction have originally been recorded in the books of Blue & Gold Pty Ltd in...
-
Y3K, Inc., has sales of $5,987, total assets of $2,532, and a debt-equity ratio of .57. If its return on equity is 11 percent, what is its net income?
-
Hemoglobin is the protein that transports oxygen in mammals. Hemoglobin is 0.347% Fe by mass, and each hemoglobin molecule contains four iron atoms. Calculate the molar mass of hemoglobin.
-
Aluminum metal is produced by passing an electric current through a solution of aluminum oxide (Al 2 O 3 ) dissolved in molten cryolite (Na 3 AlF 6 ). Calculate the molar masses of Al 2 O 3 and Na 3...
-
a) Does the ruling mean that Wendy's has breached its implied warranty of merchantability? b) If a defendant has breached the implied warranty of merchantability does it mean the defendant has also...
-
Problem 1. In a study of infant birth weight and maternal factors, the newborn babies were categorized as being either small size for gestational age (N=201) or normal size (N=2089). The following...
-
Max 1 page allowed] Consider a DRAM chip of capacity 256 KB and each memory location contains 8 bits. The memory chip is organized in matrix form with equal number of rows and column for each memory...
-
find the dimensions of a notman window of perimeter 3 9 ft that will admit the greatest possible amount of light. Round answer to two decimal places
-
Consider the expression timing is everything in relation to the building of the TOMS brand. Besides the influence of recovering economic conditions and the increased affluence of potential customers,...
-
What is corporate strategy and why is it important? Choose a company with which you are familiar, and evaluate its corporate strategy, especially in regards to financial strategies. What are some...
-
What are some possible objectives of surface modification processes?
-
For the following arrangements, discuss whether they are 'in substance' lease transactions, and thus fall under the ambit of IAS 17.
-
Which of the noncyclic isomers of C 4 H 7 F are optically active?
-
A common shorthand notation to draw organic structures is to use lines to represent CC bonds. For example, the shorthand notation for the two structural isomers of the formula C 4 H 10 are: At the...
-
Why is glycine not optically active?
-
Alphabet Company, which uses the periodic inventory method, purchases different letters for resale. Alphabet had no beginning inventory. It purchased A thru G in January at $7.50 per letter. In...
-
what is the maximum number of custom fields you can add to sales form in QB online?
-
3. Write down the names of items which are not eligible for claiming input tax credit by makingreference to the provisions of the VAT & SD Act, 2012.

Study smarter with the SolutionInn App


