Calculate the molar mass of the following substances. a. H ON c. (NH4)2Cr2O7 b. HZ
Question:
Calculate the molar mass of the following substances.
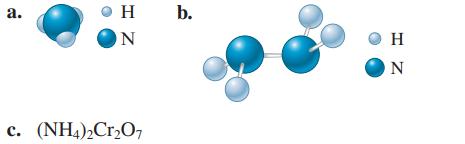
Transcribed Image Text:
a. H ON c. (NH4)2Cr2O7 b. Ο Η HZ N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a NH3 N 14 H 10 1413 17 gmol Molar mass NH...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Calculate the molar mass of the following substances: (a) Li2 CO3, (b) CS2, (c) CHCl3 (chloroform), (d) C6H8O6 (ascorbic acid, or vitamin C), (e) KNO3, (f) Mg3N2.
-
Elaborate figures given below Price per pair (dollars) 105 90 75 60 45 30 15 0 10 20 30 I E Equilibrium IS 40 50 60 70 Quantity of tennis shoes (thousands of pairs per year) 80 90 100 D
-
Richards and Willard determined the molar mass of lithium collected the following data. 6 (a) Find the mean molar mass determined by these workers (b) Find the median molar mass (c) Assuming that the...
-
Fat Gram Calculator Write a flowgorithm program that perform the following tasks: . Ask the user to input the total number of calories in the food the total fat grams in the food Display the...
-
Compute the amount that can be borrowed under each of the following circumstances: 1. A promise to repay $90,000 seven years from now at an interest rate of 6%. 2. An agreement made on February 1,...
-
Copy the Mike Owjai Manufacturing financial statements from Problem 1 in Chapter 2 into a new workbook. a. Set up a ratio worksheet similar to the one in Exhibit 4-6, page 131, and calculate all of...
-
LO5 Rikki has the following capital gains and losses for the current year: What is the effect of the capital gains and losses on Rikkis taxable income and her income tax liability? Assume that Rikki...
-
Georgia Exchange Company completed the following long-term investment transactions during 2012: At year-end the fair value of the Sydney stock is $30,900. The fair value of the Portland stock is...
-
Save Homework: Chapter 3 Homework Score: 0 of 1 pt 2 of 6 (4 complete) HW Score: 66.67%, 4 of 6 pts P3-2 (similar to) 3 Question Help (Working with the income statement) At the end of its third year...
-
Hillside issues $4,000,000 of 6%, 15-year bonds dated January 1, 2017, that pay interest semiannually on June 30 and December 31. The bonds are issued at a price of $3,456,448. Required 1. Prepare...
-
Aluminum metal is produced by passing an electric current through a solution of aluminum oxide (Al 2 O 3 ) dissolved in molten cryolite (Na 3 AlF 6 ). Calculate the molar masses of Al 2 O 3 and Na 3...
-
A diamond contains 5.0 10 21 atoms of carbon. What amount (moles) of carbon and what mass (grams) of carbon are in this diamond?
-
Ogden Industries purchased the following assets and constructed a building as well. All this was done during the current year. Assets 1 and 2 These assets were purchased as a lump sum for $186,000...
-
Small Sample Weights of M&M plain candies are normally distributed. Twelve M&M plain candies are randomly selected and weighed, and then the mean of this sample is calculated. Is it correct to...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
The Tokyo Olympics After watching How the Tokyo Olympics Became the Most Expensive Summer Game Ever video answer the following questions. * * The numbers can be made up . I just need help with an...
-
Shamika conducts a study comparing the performance of individuals on the first quiz in each of two classes taken in the first term of their freshman year. The scores, out of 100, are provided for...
-
Adult Sleep Times (hours) of sleep for randomly selected adult subjects included in the National Health and Nutrition Examination Study are listed below. Here are the statistics for this sample: n =...
-
KC Stern, a design engineer for Boeing Commercial Airplane Group, is doing some design work and has a question regarding the hole size that is drilled before tapping internal threads. The book...
-
Show that gj concave AHUCQ Abadie For nonnegative variables, we have the following corollary.
-
In general terms, what does the secondary structure of a protein represent? How is the secondary structure of a protein related to its function?
-
When pure crystalline amino acids are heated, decomposition generally occurs before the solid melts. Account for this observation.
-
When toluene (C 6 H 5 CH 3 ) reacts with chlorine gas in the presence of iron(III) catalyst, the product is a mixture of the ortho and para isomers of C 6 H 4 ClCH 3 . However, when the reaction is...
-
Incorrect answer iconYour answer is incorrect. On January 1, 2021, Crane Corporation had 1,030,000 shares of common stock outstanding. On March 1, the corporation issued 120,000 new shares to raise...
-
What is the net book value of a non current asset represent? Does the network value represent the market value of the asset?
-
Question 8 (3 points) Which of the following is a "preventative control" (versus a detective control)? Taking periodic inventory. Month-end bank reconciliations. Periodically checking customer...

Study smarter with the SolutionInn App


