Consider the following electrostatic potential diagrams for some covalent compounds. Which of the represented compounds would not
Question:
Consider the following electrostatic potential diagrams for some covalent compounds. Which of the represented compounds would not be soluble in water?
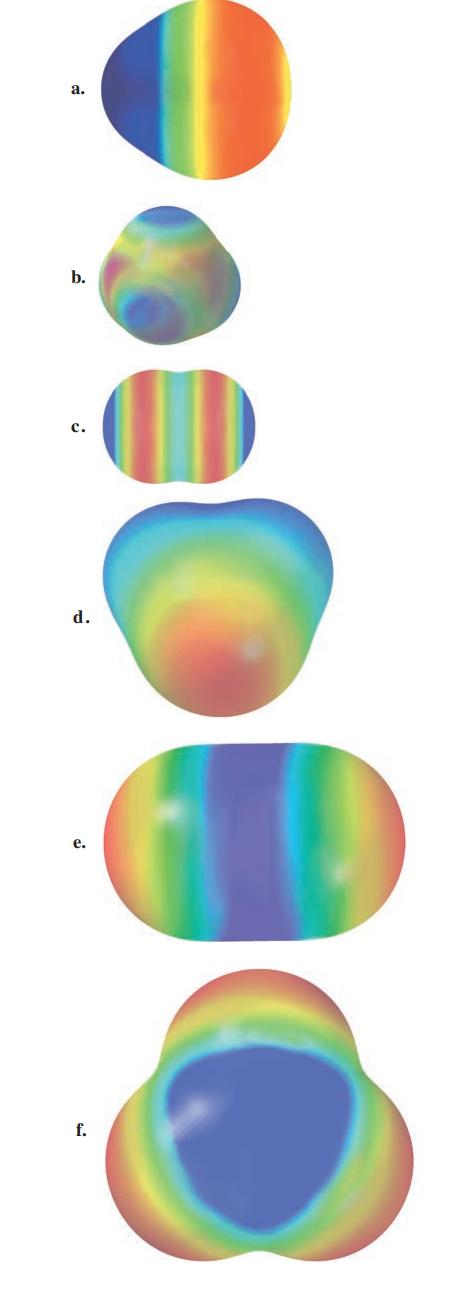
Transcribed Image Text:
a. b. C. d. e. f.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The compounds that are not soluble in water are a b c d e f and g Water is a polar molecule and it i...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Consider the following electrostatic potential diagrams: Rank the compounds from lowest to highest boiling point, and explain your answer. Ethanol Propane Acetone
-
The following electrostatic potential diagrams represent H2, HCl, or NaCl. Label each, and explain your choices. (a) (b)
-
The following electrostatic potential diagrams represent CH4, NH3, or H2O. Label each, and explain your choices. a. C. b.
-
You start driving east for 14 miles, turn left, and drive north for another 10 miles. At the end of driving, what is your straight line distance from your starting point? Round to the nearest tenth...
-
Kelly Malone plans to have $50 withheld from her monthly paycheck and deposited in a savings account that earns 12% annually, compounded monthly. If Malone continues with her plan for two and...
-
Some people believe that talking on a cell phone while driving slows reaction time, increasing the risk of accidents. The study described in the paper A Comparison of the Cell Phone Driver and the...
-
LO6,7 Determine how much interest income Later Federal Loan Company, a cash basis taxpayer, must recognize on each of the following loans in 2010: a. A $10,000, 8.5%, 6-month loan made on October 1,...
-
Magnetic-Optical Corporation offers a variety of share-based compensation plans to employees. Under its restricted stock award plan, the company on January 1, 2011, granted 4 million of its $1 par...
-
You have the following information on ABC Inc. and XYZ Co. Profit Margin 4% Asset Turnover 2 0.8 Equity Multiplier 1.5 3 ABC XYZ 6% a) Calculate both companies' return on equity b) Calculate the...
-
Table 1 MACRS Half-Year Convention Depreciation Rate for Recovery Period 3-Year 5-Year 7-Year 10-Year 15-Year 20-Year Year 1 Year 2 33.33% 20.00% 14.29% 10.00% 5.00% 3.750% 44.45 32.00 24.49 18.00...
-
Consider the steps involved in balancing oxidation reduction reactions by using oxidation states. The key to the oxidation states method is to equalize the electrons lost by the species oxidized with...
-
What is an acidbase reaction? Strong bases are soluble ionic compounds that contain the hydroxide ion. List the strong bases. When a strong base reacts with an acid, what is always produced? Explain...
-
a. The residual distribution policy is based on the premise that, since new common stock is more costly than retained earnings, a firm should use all the retained earnings it can to satisfy its...
-
Ash purchases 500 shares of XYZ for $10/share. Ten months later, when the shares are trading at $15/share, they donate them to Caring Trust, a qualified charity. Three months after the donation is...
-
Bob gets a X = 60 on his psychology exam and a X = 56 on his Biology exam. Psych exam scores had a =50 and =10 while Bio exam scores had a =48 and =4. Both professors grade on a curve. 1 - For which...
-
1. Lucky Company's direct labor information for the month of February is as follows: Actual direct labor hours worked (AQ) 61,500 Standard direct labor hours allowed (SQ) 63,000 Total payroll for...
-
MAT 152 Project 3: MLB Team Salaries The data set below is the total salary of each Major League Baseball (MLB) team salaries per team in 2016. Find the probabilities for normal distributions and...
-
deficit, surplusincreased, decreased$795, $1,975, $54,635, $35 6. Cash-flow statement Sam and Joan Wallingford have been married for two years. They have been trying to save but feel that there is...
-
What are some of the reasons that paints may be specified for manufactured items?
-
As water moves through the hydrologic cycle, water quality changes are common because of natural phenomena or anthropogenic pollution. Using Figure 11.1, describe how water-quality changes occur...
-
Consider element 113, Nh. What is the expected electron configuration for Nh? What oxidation states would be exhibited by Nh in its compounds?
-
Many lithium salts are hygroscopic (absorb water), but the corresponding salts of the other alkali metals are not. Why are lithium salts different from the others?
-
There are three known xenon fluoride covalent compounds: XeF 2 , XeF 4 , and XeF 6 . In general, the xenon fluoride compounds must be stored in an inert atmosphere, free of oxygen and water. Why is...
-
what is excluded from income even when cancellation of debt income must be recognized?
-
1. Explain globalization. 2. in what ways has globalization help the united state? How has the globalization hurt the United States 3. take an emerging market economy like jamaica, the dominican...
-
1. Annual wage: $54,500. If to contribute to company 401(k) plan per biweekly paycheck. What is the effect of the pre-tax deduction regarding the contributions listed below, a) $50 per paycheck b) 5%...

Study smarter with the SolutionInn App


