The vanadium in a sample of ore is converted to VO 2+ . The VO 2+ ion
Question:
The vanadium in a sample of ore is converted to VO2+. The VO2+ ion is subsequently titrated with MnO4- in acidic solution to form V(OH)4+ and manganese(II) ion. The unbalanced titration reaction is
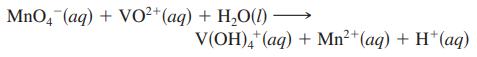
To titrate the solution, 26.45 mL of 0.02250 M MnO4- was required. If the mass percent of vanadium in the ore was 58.1%, what was the mass of the ore sample?
Transcribed Image Text:
MnO4 (aq) + VO²+ (aq) + H₂O(1) - V(OH)4+ (aq) + Mn²+ (aq) + H+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To balance the titration reaction we can use the oxidation state method MnO4 aq VO2 aq H2Ol VOH4 aq ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
A 0.5510-g sample consisting of a mixture of iron and iron(III) oxide was dissolved completely in acid to give a solution containing iron(II) and iron(III) ions. A reducing agent was added to convert...
-
The total concentration of Ca2 + and Mg2+ in a sample of hard water was determined by titrating a 0.100-L sample of the water with a solution of EDTA4-.The EDTA4- chelates the two cations: It...
-
The following data are for MarvinMarvin Department Store. The account balances (in thousands) are for 2017LOADING... (Click the icon to view the account balances.) Requirements 1. Compute (a) the...
-
Answer the Multple Choice Questions and the code for problem 6in the end PROBLEM 1: General UNIX 1. What is UNIX? a) an operating system b) a text editor c) programming language d) software program...
-
Observe the operations at your favorite fast-food restaurant. Required 1. How many people does it take to fill a typical order of sandwich, beverage, and one side-order? 2. Describe the activities...
-
A discussion of digital ethics appears in the article Academic Cheating, Aided by Cell Phones or Web, Shown to be Common (Los Angeles Times, June 17, 2009). One question posed in the article is: What...
-
RealTime Rentals leases space on its Internet server. Its standard one-year lease agreement requires new customers to pay the first and last months rent upon signing the lease and a $500 deposit that...
-
During 2016 and 2017, Agatha Corp. completed the following transactions relating to its bond issue. The corporations fiscal year is the calendar year. 2016 Jan. 1 Issued $300,000 of 10-year, 6...
-
iSeeit! Video Case: Global Entry Strategies When expanding into a global marketplace, North American companies must select a means of market entry. The decision between exporting, licensing, joint...
-
You are working on a free-form Packet Tracer challenge activity as seen in Figure 1, you have been given the London Railways network.' The purpose of this EMA question is to build upon each of the...
-
A 0.500-L sample of H 2 SO 4 solution was analyzed by taking a 100.0-mL aliquot and adding 50.0 mL of 0.213 M NaOH. After the reaction occurred, an excess of OH - ions remained in the solution. The...
-
The blood alcohol (C 2 H 5 OH) level can be determined by titrating a sample of blood plasma with an acidic potassium dichromate solution, resulting in the production of Cr 3+ (aq) and carbon...
-
What differences might you expect to see in the approach used for global manufacturing and supply chain management for a product that has stable demand and limited change in its basic design, versus...
-
Mountain Sports, Inc., is a retailer that has engaged you to assist in the preparation of its financial statements at December 31, 2021. Following are the correct adjusted account balances, in...
-
(a) sint 1-cost (b) Please note each question has 4 options: (a), (b), (c) and (d). 1. Given x = t - sint and y = 1 - cost, then dy 1-cost t-sint = 1-cost sint sint cost-1 2. Given sin x cos y - 2 =...
-
Sometimes I forget a few items when I leave the house in the morning. For example, here are probabilities that I forget various pieces of footwear: left sock 0.2 right sock 0.1 left shoe 0.1 right...
-
Sravani Bought an audio CD containing 1 0 0 audio files , 1 0 of which are by S . P . Balasubrahmanyam . Assume the shuffle option is enabled to play the songs in random order. What is the...
-
Kroger Co. is one of the largest retail food companies in the United States as measured by total annual sales. The Kroger Co. operates supermarkets, convenience stores, and manufactures and processes...
-
Why do fatigue failures almost always occur at the surface?
-
Propose a reasonable mechanism for the following reaction. OH
-
The compound Ni(H 2 O) 6 Cl 2 is green, whereas Ni(NH 3 ) 6 Cl 2 is violet. Predict the predominant color of light absorbed by each compound. Which compound absorbs light with the shorter wavelength?...
-
Oxalic acid is often used to remove rust stains. What properties of oxalic acid allow it to do this?
-
A certain first-row transition metal ion forms many different colored solutions. When four coordination compounds of this metal, each having the same coordination number, are dissolved in water, the...
-
James Bond, Inc., owns 4,000,000 shares of xyz Corp Inc. On December 31, 2012, James distributed these shares as a dividend to its shareholders. This is an example of a Select one: a. property...
-
Question 3 a.) The more days sales in inventory the higher the inventory turnover.(True/False) b.) Unearned income is a liability.(True/False) c.) Deferred revenue is unearned.(True/False) d.)...
-
A company has sales of $715,400 and cost of goods sold of $286,400. Its gross profit equals

Study smarter with the SolutionInn App


