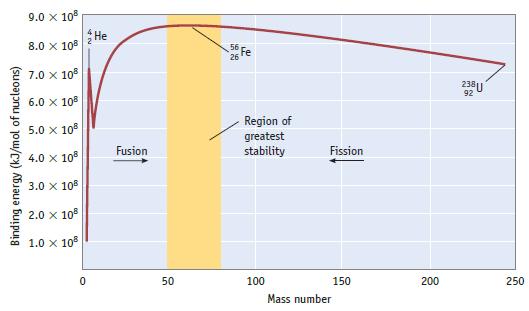
A graph of binding energy per nucleon is shown in Figure 25.4. Explain how the data used
Question:
A graph of binding energy per nucleon is shown in Figure 25.4. Explain how the data used to construct this graph were obtained.
Data given in Figure 25.4
Transcribed Image Text:
Binding energy (kJ/mol of nucleons) 9.0 x 108 8.0 x 108 7.0 x 108 6.0 x 108 5.0 X 108 4.0 x 108 3.0 X 108 2.0 x 108 1.0 x 108 0 He Fusion 50 56 Fe Region of greatest stability 100 Fission 150 Mass number 200 238U 92 250
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The data used to construct the graph of binding energy per nucleon in Figure 254 were obtained from a series of experiments that were conducted at the ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Consider the following graph of binding energy per nucleon as a function of mass number. a. What does this graph tell us about the relative halflives of the nuclides? Explain your answer. b. Which...
-
The most stable nucleus in terms of binding energy per nucleon is 56Fe. If the atomic mass of 56Fe is 55.9349 amu, calculate the bind mg energy per nucleon for 56Fe.
-
The isotope has the largest binding energy per nucleon of any isotope. Calculate this value from the atomic mass of nickel-62 (61.928345 amu) and compare it with the value given for iron-56 in Table...
-
The Black Sheep Inc.'s call option has the following characteristics: Exercise price of The Black Sheep Inc.'s call option ($) 75 Annualized risk-free rate 4.5% Time to the expiration of the options...
-
Titanium Metals Inc. casts blades for turbine engines. Within the Casting Department, alloy is first melted in a crucible, then poured into molds to produce the castings. On October 1, there were 700...
-
The following accounting equation worksheet shows the transactions for Internet Advertising, a corporation, for the first month of business October 2009. Requirements 1. Analyze each transaction in...
-
Are there other ways in which managers can try to prevent or reduce potentially disruptive conflicts?
-
For the year ended December 31, 2012, the job cost sheets of Dosey Company contained the following data. Other data: 1. Raw materials inventory totaled $20,000 on January 1. During the year, $100,000...
-
Waterway Beauty Corporation manufactures cosmetic products that are sold through a network of sales agents. The agents are paida commission of 21% of sales. The income statement for the year ending...
-
What information was used to identify and particles?
-
Rank the three types of natural radiation (, , ): (a) In order of increasing mass (b) In order of increasing penetrating power
-
Insurance Coverage. PAJ, Inc., a jewelry company, had a commercial general liability (CGL) policy from Hanover Insurance Co. It covered, among other things, liability for advertising injury. The...
-
If WHO, the World Health Organization,defines health as a state of completephysical, mental and social well-being and not merely the absenceof disease and infirmity (WHO, 2011)and wellness is...
-
As a manager, you want to find a way to motivate Nate and increase his engagement and job satisfaction in the workplace. Drawing upon a behavioral theory of motivation, discuss how you, as a manager,...
-
Why the sudden increase in income before taxes in 2021? 8. Why were the operating assets the highest in 2019? 9. Why are the short-term loans the highest in 2020? 10. Why are the other long-term...
-
Mercy wants to make sure that she will be able to provide for her daughter's college and plans to open a savings account with a bank that is ready to pay interest as shown below per year compounded...
-
Question 1. For a firm that uses portfolio management, please give a real or hypothetical example of how the CEO's personal bases for power help organizational performance. Question 2. Give a real...
-
Let z be the n à 1 column vector all of whose entries are equal to 1. (a) Show that if A is an m à n matrix, the ith entry of the product v = Az is the ith row sum of A, meaning the sum...
-
The company manufactures three products: wooden chairs, tables and dressers. AFC started off as a 'Mom & Pop' shop but has grown rapidly. AFC uses one assembly line to build all three products,...
-
Find i and V o in the circuit of Fig. 2.100 . 80 2 24 Q ww 25 2 30 : 20 20 V V. 20 2 60
-
Calculate V o and I o in the circuit of Fig. 2.99. 30 70 200 V 20 5
-
Using series/parallel resistance combination, find the equivalent resistance seen by the source in the circuit of Fig. 2.98 . Find the overall absorbed power by the resistor network. 70 2 50 2 150 2...
-
help please! Requirement 2. Journalize any required entries from the bank reconciliation. (Record debits first, then credits. Se Begin with the EFT collection, Date Credit Debit Accounts and...
-
Pear, an individual, plans to start a small business, which will operate as a corporation. In year 0, she expects the corporation to generate an ordinary loss of $1,550,000. Subsequently, she expects...
-
Daryl Kearns saved $260,000 during the 25 years that he worked for a major corporation. Now he has retired at the age of 50 and has begun to draw a comfortable pension check every month. He wants to...

Study smarter with the SolutionInn App


