A herbicide contains 2,4-D (2,4-dichlorophenoxyacetic acid), C 8 H 6 Cl 2 O 3 . A 1.236-g
Question:
A herbicide contains 2,4-D (2,4-dichlorophenoxyacetic acid), C8H6Cl2O3. A 1.236-g sample of the herbicide was decomposed to liberate the chlorine as Cl− ion. This was precipitated as AgCl, with a mass of 0.1840 g. What is the mass percent of 2,4-D in the sample?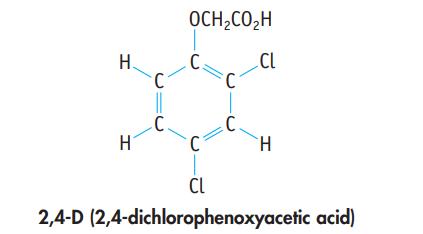
Transcribed Image Text:
H. H C C. OCH₂CO₂H C. C C. CL H CL 2,4-D (2,4-dichlorophenoxyacetic acid)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To find the mass percent of 24D in the sample you need to first calculate the molar mass of 24D and ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
This activity has the purpose of helping students explain the purposes and uses of measures of employee performance and identify the systematic way of integrated components which comprise an overall...
-
Files Links: https://drive.google.com/drive/folders/10CssG86ruVkS1bwe-B7VIpwBx2twS6n-?usp=share_link https://drive.google.com/drive/folders/10CssG86ruVkS1bwe-B7VIpwBx2twS6n-?usp=share_link You will...
-
An airplane typically approaches a runway for landing at a descent angle of 3. However, a jet experiences problems and makes an emergency descent through the air at an angle of 10. If this jet is...
-
Macmillan Learning A Geiger-Muller tube is a type gas-filled radiation detector. It can detect particles like X-rays, alpha particles, and beta rays (electrons). This is useful in quantizing the...
-
Explain the differences among factor rating, the loaddistance model, and break-even analysis. What criteria does each method use to make the location decision?
-
Worldwide, over a billion solder balls must be manufactured daily for assembling electronics packages. The uniform droplet spray method uses a piezoelectric device to vibrate a shaft in a pot of...
-
Consider the following hypothesis test. a. What is your conclusion if n1 21, 8.2, n2 26, and 4.0? Use .05 and the p-value approach. b. Repeat the test using the critical value approach. Applications
-
FASB Statement of Financial Accounting Concepts No. 2 indicates several qualitative characteristics of useful accounting information. Following is a list of some of these qualities, as well as a list...
-
Periodic Inventory Using FIFO, LIFO, and Weighted Average Cost Methods The units of an item available for sale during the year were as follows: Jan. 1 Inventory 12 units at $45 $540 Aug. 13 Purchase...
-
Sulfuric acid is listed in a catalog with a concentration of 9598%. A bottle of the acid in the stockroom states that 1.00 L has a mass of 1.84 kg. To determine the concentration of sulfuric acid in...
-
Thioridazine, C 21 H 26 N 2 S 2 , is a pharmaceutical agent used to regulate dopamine. (Dopamine, a neurotransmitter, affects brain processes that control movement, emotional response, and ability to...
-
Shankman Corporation had two issues of securities outstanding: common shares and an 8% convertible bond issue in the face amount of $8 million. Interest payment dates of the bond issue are June 30...
-
Let two planes be given by 2x-y+z = 8 and z = x+y-5 (a) Find the angle between the two planes. Leave your answer in degrees and round to the nearest tenth. (b) Find the vector equation of the line of...
-
9-2. The profile of a gear tooth shown in Fig. P9.2 is approximated by the trigonometric equation y(x) = a. Estimate the area A using eight rectangles of equal width A x = 1/8, b. Calculate the exact...
-
tube is hinged to a rotating base as shown in Fig. 4. At the instant shown, the base rotates about the z axis with a constant angular velocity ! 1 = 2 rad/s. At the same instant, the 2 tube rotates...
-
Find the limit analytically. -7x2+5x-10 lim 0 9x+13x+11 Find the limit analytically. lim +80 4x-13 5x+6x-11
-
Write a recursive function for the running time T(n) of the function given below. Prove using the iterative method that T(n) = (n). function( int n) { if(n=1) return; for(int i = 1; i
-
Eagle Golf Co. prepaid 3 years' rent ($6,000) on January 1. At December 31, Eagle prepared a trial balance and then made the necessary adjusting entry at the end of the year. Eagle adjusts its...
-
Write a paper detailing a geographic information system (GIS) of your own design that would utilize data in an original manner.
-
Draw the product for each of the following S N 2 reactions: (a) (S)-2-Chloropentane and NaSH (b) (R)-3-Iodohexane and NaCl (c) (R)-2-Bromohexane and sodium hydroxide
-
When (S)-1-bromo-1-fluoroethane reacts with sodium methoxide, an S N 2 reaction takes place in which the bromine atom is replaced by a methoxy group (OMe). The product of this reaction is...
-
Draw the transition state for each of the following S N 2 reactions: (a) (b) (c) (d) Br Br P
-
Aliara Corporation is considering purchasing one of two new machines. Estimates for each machine are as follows: Machine A Machine B Investment $107,800 $156,000 Estimated life 8 years 8 years...
-
XYZ Inc. is a manufacturer of specialized equipment which offers a leasing alternative. Provide journal entries in the books of lessor. The data relative to a typical lease are as follows: 1. The...
-
Inventory information for Part 311 of Sunland Corp. discloses the following information for the month of June. June 1 Balance 303 units @ $14 June 10 Sold 203 units @ $33 11 Purchased 796 units @ $17...

Study smarter with the SolutionInn App


